Chlorine Dioxide Melting And Boiling Point . The gas is toxic by inhalation. The large structures (the metal oxides and silicon dioxide) have high melting and boiling points because a large. The melting point of the hydrate is around 30 °f. In chlorine(vii) oxide, the chlorine uses all of its seven outer electrons in bonds with oxygen. If it should thaw and further warm up, chlorine dioxide gas is given off. This produces a much bigger molecule, and so you would expect its melting point. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will have a lower. This property makes chlorine dioxide a gas at room temperature. The gas and liquid are violently.
from www.chegg.com
The gas and liquid are violently. In chlorine(vii) oxide, the chlorine uses all of its seven outer electrons in bonds with oxygen. The large structures (the metal oxides and silicon dioxide) have high melting and boiling points because a large. This property makes chlorine dioxide a gas at room temperature. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will have a lower. The melting point of the hydrate is around 30 °f. The gas is toxic by inhalation. This produces a much bigger molecule, and so you would expect its melting point. If it should thaw and further warm up, chlorine dioxide gas is given off.
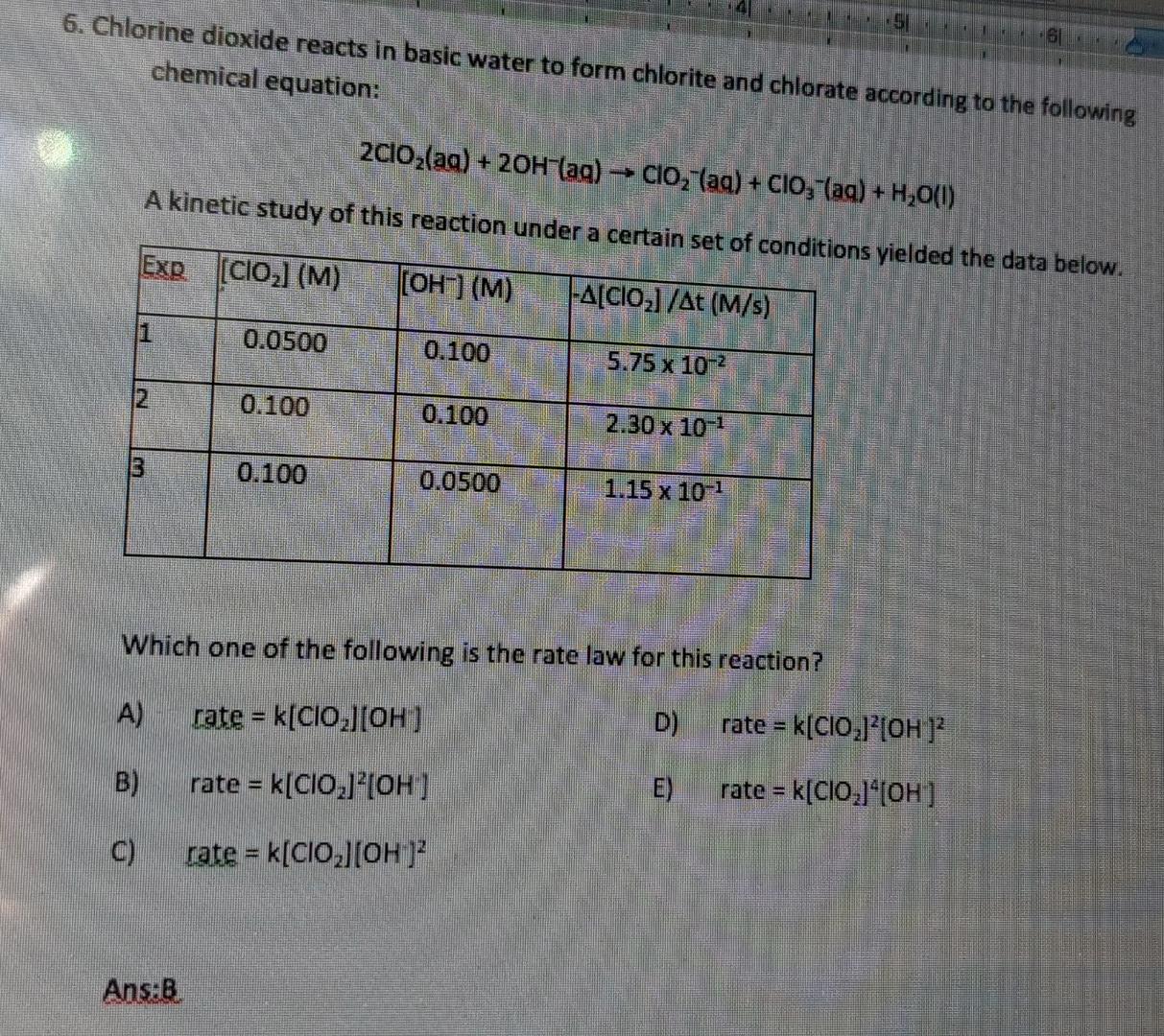
Solved 6. Chlorine dioxide reacts in basic water to form
Chlorine Dioxide Melting And Boiling Point The gas is toxic by inhalation. The large structures (the metal oxides and silicon dioxide) have high melting and boiling points because a large. The melting point of the hydrate is around 30 °f. If it should thaw and further warm up, chlorine dioxide gas is given off. In chlorine(vii) oxide, the chlorine uses all of its seven outer electrons in bonds with oxygen. The gas and liquid are violently. The gas is toxic by inhalation. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will have a lower. This produces a much bigger molecule, and so you would expect its melting point. This property makes chlorine dioxide a gas at room temperature.
From mavink.com
Chlorine Dioxide Structure Chlorine Dioxide Melting And Boiling Point Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will have a lower. In chlorine(vii) oxide, the chlorine uses all of its seven outer electrons in bonds with oxygen. The gas is toxic by inhalation. If it should thaw and further warm up, chlorine dioxide gas is given off. This property. Chlorine Dioxide Melting And Boiling Point.
From www.slideserve.com
PPT Characteristic Properties of the Halogens PowerPoint Presentation Chlorine Dioxide Melting And Boiling Point Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will have a lower. The gas and liquid are violently. The large structures (the metal oxides and silicon dioxide) have high melting and boiling points because a large. This produces a much bigger molecule, and so you would expect its melting point.. Chlorine Dioxide Melting And Boiling Point.
From chem.libretexts.org
Fundamentals of Phase Transitions Chemistry LibreTexts Chlorine Dioxide Melting And Boiling Point The melting point of the hydrate is around 30 °f. The large structures (the metal oxides and silicon dioxide) have high melting and boiling points because a large. This property makes chlorine dioxide a gas at room temperature. In chlorine(vii) oxide, the chlorine uses all of its seven outer electrons in bonds with oxygen. This produces a much bigger molecule,. Chlorine Dioxide Melting And Boiling Point.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox Chlorine Dioxide Melting And Boiling Point This produces a much bigger molecule, and so you would expect its melting point. The gas is toxic by inhalation. The gas and liquid are violently. If it should thaw and further warm up, chlorine dioxide gas is given off. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will have. Chlorine Dioxide Melting And Boiling Point.
From www.haikudeck.com
The Element Chlorine by Mary Won Chlorine Dioxide Melting And Boiling Point Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will have a lower. The melting point of the hydrate is around 30 °f. The large structures (the metal oxides and silicon dioxide) have high melting and boiling points because a large. If it should thaw and further warm up, chlorine dioxide. Chlorine Dioxide Melting And Boiling Point.
From slideplayer.com
Properties of Matter Chapter 4 ppt download Chlorine Dioxide Melting And Boiling Point The gas is toxic by inhalation. The gas and liquid are violently. The melting point of the hydrate is around 30 °f. This property makes chlorine dioxide a gas at room temperature. If it should thaw and further warm up, chlorine dioxide gas is given off. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals. Chlorine Dioxide Melting And Boiling Point.
From www.chegg.com
Solved Refer to the phase diagram for CO2. Determine its Chlorine Dioxide Melting And Boiling Point If it should thaw and further warm up, chlorine dioxide gas is given off. The large structures (the metal oxides and silicon dioxide) have high melting and boiling points because a large. The gas is toxic by inhalation. The gas and liquid are violently. In chlorine(vii) oxide, the chlorine uses all of its seven outer electrons in bonds with oxygen.. Chlorine Dioxide Melting And Boiling Point.
From www.numerade.com
SOLVED Consider the halogens chlorine, bromine, and iodine. The Chlorine Dioxide Melting And Boiling Point If it should thaw and further warm up, chlorine dioxide gas is given off. The melting point of the hydrate is around 30 °f. The gas and liquid are violently. This produces a much bigger molecule, and so you would expect its melting point. The gas is toxic by inhalation. In chlorine(vii) oxide, the chlorine uses all of its seven. Chlorine Dioxide Melting And Boiling Point.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Chlorine Dioxide Melting And Boiling Point The large structures (the metal oxides and silicon dioxide) have high melting and boiling points because a large. In chlorine(vii) oxide, the chlorine uses all of its seven outer electrons in bonds with oxygen. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will have a lower. The melting point of. Chlorine Dioxide Melting And Boiling Point.
From www.linkedin.com
Disinfection Mechanism of Chlorine Dioxide (CIO2) Chlorine Dioxide Melting And Boiling Point This produces a much bigger molecule, and so you would expect its melting point. The gas is toxic by inhalation. The large structures (the metal oxides and silicon dioxide) have high melting and boiling points because a large. The melting point of the hydrate is around 30 °f. If it should thaw and further warm up, chlorine dioxide gas is. Chlorine Dioxide Melting And Boiling Point.
From hxewsjoxk.blob.core.windows.net
Boiling Point Chlorine Methane at Mary Suttles blog Chlorine Dioxide Melting And Boiling Point This produces a much bigger molecule, and so you would expect its melting point. This property makes chlorine dioxide a gas at room temperature. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will have a lower. The gas and liquid are violently. If it should thaw and further warm up,. Chlorine Dioxide Melting And Boiling Point.
From material-properties.org
Chlorine Thermal Properties Melting Point Thermal Conductivity Chlorine Dioxide Melting And Boiling Point Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will have a lower. The gas is toxic by inhalation. This property makes chlorine dioxide a gas at room temperature. The melting point of the hydrate is around 30 °f. In chlorine(vii) oxide, the chlorine uses all of its seven outer electrons. Chlorine Dioxide Melting And Boiling Point.
From guidemanualeruptivity.z14.web.core.windows.net
Chemistry Boiling Point Chart Chlorine Dioxide Melting And Boiling Point The gas and liquid are violently. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will have a lower. The large structures (the metal oxides and silicon dioxide) have high melting and boiling points because a large. The melting point of the hydrate is around 30 °f. This property makes chlorine. Chlorine Dioxide Melting And Boiling Point.
From www.slideserve.com
PPT Objectives PowerPoint Presentation, free download ID5858440 Chlorine Dioxide Melting And Boiling Point The melting point of the hydrate is around 30 °f. In chlorine(vii) oxide, the chlorine uses all of its seven outer electrons in bonds with oxygen. The gas is toxic by inhalation. The large structures (the metal oxides and silicon dioxide) have high melting and boiling points because a large. This property makes chlorine dioxide a gas at room temperature.. Chlorine Dioxide Melting And Boiling Point.
From wisc.pb.unizin.org
M10Q2 Melting and Boiling Point Comparisons Chem 103/104 Resource Book Chlorine Dioxide Melting And Boiling Point In chlorine(vii) oxide, the chlorine uses all of its seven outer electrons in bonds with oxygen. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will have a lower. The gas is toxic by inhalation. The gas and liquid are violently. This produces a much bigger molecule, and so you would. Chlorine Dioxide Melting And Boiling Point.
From www.chegg.com
Solved 2. Chlorine has a melting point of 168K. Chlorine Chlorine Dioxide Melting And Boiling Point The large structures (the metal oxides and silicon dioxide) have high melting and boiling points because a large. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will have a lower. The gas and liquid are violently. This produces a much bigger molecule, and so you would expect its melting point.. Chlorine Dioxide Melting And Boiling Point.
From www.chegg.com
Solved 6. Chlorine dioxide reacts in basic water to form Chlorine Dioxide Melting And Boiling Point The gas is toxic by inhalation. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will have a lower. In chlorine(vii) oxide, the chlorine uses all of its seven outer electrons in bonds with oxygen. This property makes chlorine dioxide a gas at room temperature. This produces a much bigger molecule,. Chlorine Dioxide Melting And Boiling Point.
From www.tradeindia.com
Liquid Chlorine Dioxide Boiling Point 9.7 A C at Best Price in Surat Chlorine Dioxide Melting And Boiling Point The melting point of the hydrate is around 30 °f. This property makes chlorine dioxide a gas at room temperature. In chlorine(vii) oxide, the chlorine uses all of its seven outer electrons in bonds with oxygen. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will have a lower. This produces. Chlorine Dioxide Melting And Boiling Point.
From www.dreamstime.com
3D Image of Chlorine Dioxide Skeletal Formula Stock Illustration Chlorine Dioxide Melting And Boiling Point The large structures (the metal oxides and silicon dioxide) have high melting and boiling points because a large. The gas is toxic by inhalation. If it should thaw and further warm up, chlorine dioxide gas is given off. This property makes chlorine dioxide a gas at room temperature. In chlorine(vii) oxide, the chlorine uses all of its seven outer electrons. Chlorine Dioxide Melting And Boiling Point.
From resource.studiaacademy.com
2.2 Group 7 (Halogens) Chlorine, Bromine and Iodine Studia Academy Chlorine Dioxide Melting And Boiling Point The melting point of the hydrate is around 30 °f. The gas is toxic by inhalation. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will have a lower. The large structures (the metal oxides and silicon dioxide) have high melting and boiling points because a large. In chlorine(vii) oxide, the. Chlorine Dioxide Melting And Boiling Point.
From www.slideshare.net
The periodic table and identification of ions Chlorine Dioxide Melting And Boiling Point The gas is toxic by inhalation. The melting point of the hydrate is around 30 °f. This property makes chlorine dioxide a gas at room temperature. The gas and liquid are violently. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will have a lower. This produces a much bigger molecule,. Chlorine Dioxide Melting And Boiling Point.
From www.nuclear-power.com
Chlorine Melting Point Boiling Point Chlorine Dioxide Melting And Boiling Point The large structures (the metal oxides and silicon dioxide) have high melting and boiling points because a large. The gas and liquid are violently. The gas is toxic by inhalation. This property makes chlorine dioxide a gas at room temperature. If it should thaw and further warm up, chlorine dioxide gas is given off. This produces a much bigger molecule,. Chlorine Dioxide Melting And Boiling Point.
From www.numerade.com
SOLVED Chlorine dioxide reacts in basic water to form chlorite and Chlorine Dioxide Melting And Boiling Point If it should thaw and further warm up, chlorine dioxide gas is given off. In chlorine(vii) oxide, the chlorine uses all of its seven outer electrons in bonds with oxygen. This property makes chlorine dioxide a gas at room temperature. The melting point of the hydrate is around 30 °f. This produces a much bigger molecule, and so you would. Chlorine Dioxide Melting And Boiling Point.
From www.slideshare.net
Chem matters ch7_covalent_bonding Chlorine Dioxide Melting And Boiling Point The gas is toxic by inhalation. This property makes chlorine dioxide a gas at room temperature. If it should thaw and further warm up, chlorine dioxide gas is given off. In chlorine(vii) oxide, the chlorine uses all of its seven outer electrons in bonds with oxygen. The gas and liquid are violently. Chlorine, cl 2, is a much smaller molecule. Chlorine Dioxide Melting And Boiling Point.
From slideplayer.com
Chapter 8 “Covalent Bonding” ppt download Chlorine Dioxide Melting And Boiling Point In chlorine(vii) oxide, the chlorine uses all of its seven outer electrons in bonds with oxygen. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will have a lower. This property makes chlorine dioxide a gas at room temperature. If it should thaw and further warm up, chlorine dioxide gas is. Chlorine Dioxide Melting And Boiling Point.
From www.slideserve.com
PPT Introduction to Chlorine Dioxide Technology PowerPoint Chlorine Dioxide Melting And Boiling Point Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will have a lower. If it should thaw and further warm up, chlorine dioxide gas is given off. This produces a much bigger molecule, and so you would expect its melting point. This property makes chlorine dioxide a gas at room temperature.. Chlorine Dioxide Melting And Boiling Point.
From www.differencebetween.com
Difference Between Chlorine and Chlorine Dioxide Compare the Chlorine Dioxide Melting And Boiling Point The melting point of the hydrate is around 30 °f. This produces a much bigger molecule, and so you would expect its melting point. In chlorine(vii) oxide, the chlorine uses all of its seven outer electrons in bonds with oxygen. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will have. Chlorine Dioxide Melting And Boiling Point.
From socratic.org
What is the relation between critical temperature and boiling point or Chlorine Dioxide Melting And Boiling Point This property makes chlorine dioxide a gas at room temperature. The melting point of the hydrate is around 30 °f. In chlorine(vii) oxide, the chlorine uses all of its seven outer electrons in bonds with oxygen. The large structures (the metal oxides and silicon dioxide) have high melting and boiling points because a large. The gas is toxic by inhalation.. Chlorine Dioxide Melting And Boiling Point.
From fyouzbxlm.blob.core.windows.net
Why Does Chlorine Boiling Point at Hector Duerr blog Chlorine Dioxide Melting And Boiling Point The melting point of the hydrate is around 30 °f. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will have a lower. If it should thaw and further warm up, chlorine dioxide gas is given off. The gas is toxic by inhalation. This produces a much bigger molecule, and so. Chlorine Dioxide Melting And Boiling Point.
From peacecommission.kdsg.gov.ng
Oxygen Boiling Point Chlorine Dioxide Melting And Boiling Point The large structures (the metal oxides and silicon dioxide) have high melting and boiling points because a large. This produces a much bigger molecule, and so you would expect its melting point. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will have a lower. The gas and liquid are violently.. Chlorine Dioxide Melting And Boiling Point.
From www.chegg.com
Solved Explain for each observation with appropriate atomic Chlorine Dioxide Melting And Boiling Point This produces a much bigger molecule, and so you would expect its melting point. In chlorine(vii) oxide, the chlorine uses all of its seven outer electrons in bonds with oxygen. The large structures (the metal oxides and silicon dioxide) have high melting and boiling points because a large. Chlorine, cl 2, is a much smaller molecule with comparatively weak van. Chlorine Dioxide Melting And Boiling Point.
From slideplayer.com
Predicting and Identifying Reactions and Products ppt download Chlorine Dioxide Melting And Boiling Point Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will have a lower. If it should thaw and further warm up, chlorine dioxide gas is given off. The large structures (the metal oxides and silicon dioxide) have high melting and boiling points because a large. This produces a much bigger molecule,. Chlorine Dioxide Melting And Boiling Point.
From www.reddit.com
Pressure of a liquid gas? r/chemistry Chlorine Dioxide Melting And Boiling Point The large structures (the metal oxides and silicon dioxide) have high melting and boiling points because a large. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals attractions, and so chlorine will have a lower. The gas is toxic by inhalation. In chlorine(vii) oxide, the chlorine uses all of its seven outer electrons in bonds. Chlorine Dioxide Melting And Boiling Point.
From www.numerade.com
SOLVED Sketch the phase diagram of oxygen, 0z, from the following Chlorine Dioxide Melting And Boiling Point This produces a much bigger molecule, and so you would expect its melting point. In chlorine(vii) oxide, the chlorine uses all of its seven outer electrons in bonds with oxygen. The gas and liquid are violently. If it should thaw and further warm up, chlorine dioxide gas is given off. The melting point of the hydrate is around 30 °f.. Chlorine Dioxide Melting And Boiling Point.
From fyouzbxlm.blob.core.windows.net
Why Does Chlorine Boiling Point at Hector Duerr blog Chlorine Dioxide Melting And Boiling Point This property makes chlorine dioxide a gas at room temperature. This produces a much bigger molecule, and so you would expect its melting point. The gas is toxic by inhalation. In chlorine(vii) oxide, the chlorine uses all of its seven outer electrons in bonds with oxygen. Chlorine, cl 2, is a much smaller molecule with comparatively weak van der waals. Chlorine Dioxide Melting And Boiling Point.