Medical Device Definitions . A medical device includes devices intended to administer a medicinal product or which incorporate as an integral part a. In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as.
from mungfali.com
A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. A medical device includes devices intended to administer a medicinal product or which incorporate as an integral part a. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose.
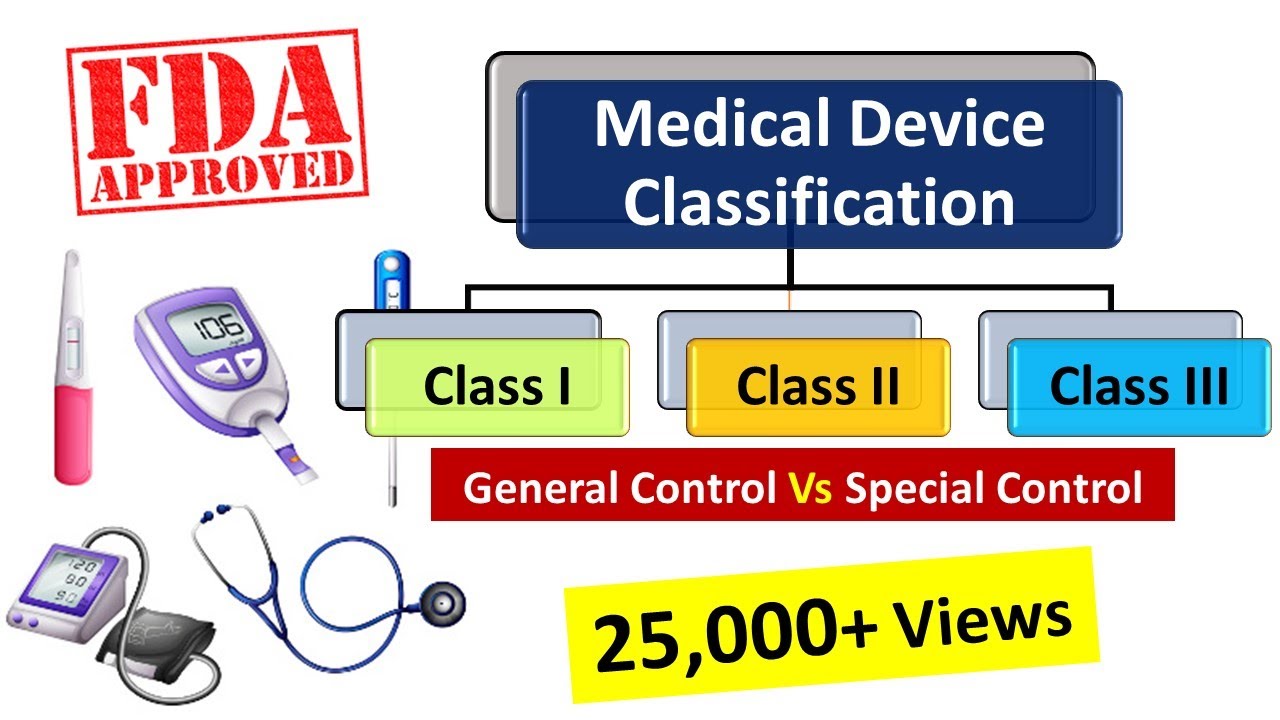
Classification Of Medical Devices
Medical Device Definitions A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. A medical device includes devices intended to administer a medicinal product or which incorporate as an integral part a.
From www.slideserve.com
PPT Life cycle of medical devices PowerPoint Presentation ID254282 Medical Device Definitions Medical devices are products or equipment intended for a medical purpose. A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. A medical device includes devices intended to administer a medicinal. Medical Device Definitions.
From spyro-soft.com
The Complete Guide to EU Medical Device Regulation Spyrosoft Medical Device Definitions In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,.. Medical Device Definitions.
From www.pinterest.com
What is a Medical Device? (Official definition for EU, USA, China Medical Device Definitions A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a. Medical Device Definitions.
From mavink.com
Medical Device Life Cycle Phases Medical Device Definitions Medical devices are products or equipment intended for a medical purpose. A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. In the european union (eu) they must undergo a conformity. A medical device includes devices intended to administer a medicinal product or which incorporate as an integral part a. A medical device. Medical Device Definitions.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Medical Device Definitions A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. Medical devices are products or equipment intended for a medical purpose. A medical device includes devices intended. Medical Device Definitions.
From medicaldevicehq.com
What is a medical device? Medical Device HQ Medical Device Definitions A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. A medical device includes devices intended to administer a medicinal product or which incorporate as an integral part a. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. A medical. Medical Device Definitions.
From www.qualitymeddev.com
Medical Device Definition according to EU MDR 2017/745 Medical Device Definitions A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. Medical devices are products or equipment intended for a medical purpose. A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. Determine if your product meets the. Medical Device Definitions.
From www.slideserve.com
PPT Medical Devices PowerPoint Presentation, free download ID991833 Medical Device Definitions Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose. A medical device includes devices intended to administer a medicinal product or which incorporate as an integral. Medical Device Definitions.
From www.researchgate.net
(PDF) Medical Device scope of definition and classifications Medical Device Definitions Medical devices are products or equipment intended for a medical purpose. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. A. Medical Device Definitions.
From www.arenasolutions.com
Medical Device Definition Arena Medical Device Definitions Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for. Medical Device Definitions.
From www.greenlight.guru
Software as a Medical Device Definitions, Examples & Regulatory Framework Medical Device Definitions Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for. Medical Device Definitions.
From www.pinterest.com
Infographic on Understanding FDA Device Classes from Medical Device Definitions Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,.. Medical Device Definitions.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Definitions A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. A medical device includes devices intended to administer a medicinal product or which incorporate as an integral part a. A medical device. Medical Device Definitions.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Medical Device Definitions A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. Medical devices are products or equipment intended for a medical purpose. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. A medical device includes devices intended. Medical Device Definitions.
From de.slideshare.net
Medical device definition Medical Device Definitions Medical devices are products or equipment intended for a medical purpose. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. In. Medical Device Definitions.
From www.orielstat.com
All Class 1 Medical Device Manufacturers Must Meet These Specific EU Medical Device Definitions A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. Medical devices are products or equipment intended for a medical purpose. A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. Determine if your product meets the. Medical Device Definitions.
From spyro-soft.com
EU MDR vs FDA what are the main differences and similarities? Medical Device Definitions A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs. Medical Device Definitions.
From www.youtube.com
Classification of Medical devices / FDA regulations/ Example of Medical Medical Device Definitions Medical devices are products or equipment intended for a medical purpose. A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. In the european union (eu) they must undergo a conformity. A medical device includes devices intended to administer a medicinal product or which incorporate as an integral part a. A medical device. Medical Device Definitions.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Device Definitions A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. In the european union (eu) they must undergo a conformity. Medical devices. Medical Device Definitions.
From medicaldevicehq.com
Different classifications rules for medical device software An Medical Device Definitions In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose. A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act.. Medical Device Definitions.
From mungfali.com
Classification Of Medical Devices Medical Device Definitions Medical devices are products or equipment intended for a medical purpose. A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. A medical device includes devices intended to administer a medicinal product or which incorporate as an integral part a. In the european union (eu) they must undergo a conformity. A medical device. Medical Device Definitions.
From news.iscas.co
Interoperability standards for medical device integration in the OR and Medical Device Definitions A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. A medical device includes devices intended to administer a medicinal product or which incorporate as an integral part a. In the european union (eu) they must undergo a conformity. Determine if your product meets. Medical Device Definitions.
From laegemiddelstyrelsen.dk
Development of medical devices Medical Device Definitions A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. A medical device includes devices intended to administer a medicinal product or which. Medical Device Definitions.
From www.vrogue.co
Understanding The 7 Phases Of Medical Device Developm vrogue.co Medical Device Definitions In the european union (eu) they must undergo a conformity. A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. Medical devices are products or equipment intended for a medical purpose.. Medical Device Definitions.
From www.youtube.com
What are medical devices? YouTube Medical Device Definitions A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. Medical devices are products or equipment intended for a medical purpose. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. In the european union (eu) they. Medical Device Definitions.
From qbdgroup.com
What is a medical device? Key global definitions and regulations Medical Device Definitions A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. Medical devices are products or equipment intended for a medical purpose. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. A medical device is any instrument, apparatus, appliance, software, material,. Medical Device Definitions.
From waddellgrp.com
So you want to make a Medical Device . . . Medical Project Management Medical Device Definitions In the european union (eu) they must undergo a conformity. A medical device includes devices intended to administer a medicinal product or which incorporate as an integral part a. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. Determine if your product meets. Medical Device Definitions.
From medicaldevicehq.com
What is a medical device according to the MDR Medical Device HQ 1 Medical Device Definitions In the european union (eu) they must undergo a conformity. A medical device includes devices intended to administer a medicinal product or which incorporate as an integral part a. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. A medical device can be any instrument, apparatus, implement,. Medical Device Definitions.
From talema.com
An Introduction to Medical Electrical Devices The Talema Group Medical Device Definitions A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs a medical purpose, such as. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. A medical device includes devices intended to administer a medicinal product or. Medical Device Definitions.
From kvalito.ch
Medical Devices; US and Chinese legislation Kvalito Medical Device Definitions A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. In the european union (eu) they must undergo a conformity. A medical device includes devices intended to administer a medicinal product or which incorporate as an integral part a. A medical device is any instrument, apparatus, appliance, software, material, or other article, which. Medical Device Definitions.
From www.slideserve.com
PPT The FDA Landscape AdvaMed September 2008 PowerPoint Presentation Medical Device Definitions A medical device includes devices intended to administer a medicinal product or which incorporate as an integral part a. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a. Medical Device Definitions.
From cliniexperts.com
ISO 13485 Medical Devices Certification Medical Device ISO Standards Medical Device Definitions Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. A medical device includes devices intended to administer a medicinal product or which incorporate as an integral part a. In the european union (eu) they must undergo a conformity. A medical device can be any instrument, apparatus, implement,. Medical Device Definitions.
From es.slideshare.net
Regulation of Medical Devices in US Medical Device Definitions A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. A medical device includes devices intended to administer a medicinal product or which incorporate as an integral part a. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. Medical devices. Medical Device Definitions.
From www.statseal.com
MKTFORM008.1Medical Device Symbols Glossary StatSeal Medical Device Definitions Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. A medical device includes devices intended to administer a medicinal product or which incorporate as an integral part a. A medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use. Medical Device Definitions.
From angelanjohnson.com
Medical Devices Angela N Johnson Medical Device Definitions A medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. In the european union (eu) they must undergo a conformity. A medical device includes devices intended to administer a medicinal product or which incorporate as an integral part a. A medical device is any instrument, apparatus, appliance, software, material, or other article, which. Medical Device Definitions.