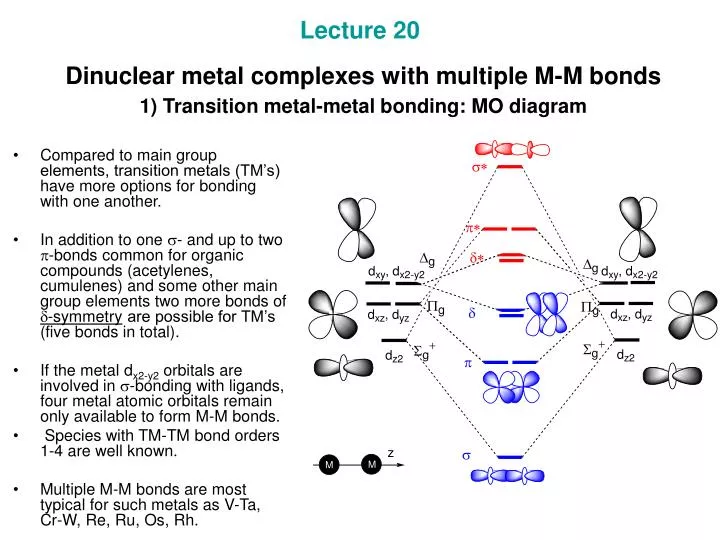
What Is A Quadruple Bond . A quadruple bond is a type of chemical bond in which four pairs of electrons are shared between two atoms, resulting in a very strong bond. A 'quadruple bond' refers to a type of bond between metal atoms where the bond order is greater than 3. High bonding order possible in main group and may be responsible for the ability to isolate molecular species. A quadruple bond is a type of chemical bond between two atoms involving eight electrons. C 2 ’s two carbon atoms aren’t joined by a double bond as usually thought, or even. A quadruple bond is a type of chemical bond between two atoms involving 8 electrons. Cx2 and its isoelectronic molecules cnx+, bn and cbx− (each having eight valence electrons) are bound by a quadruple bond. There are no arrangements which allow overlap of atomic orbitals that create four bonding molecular orbitals with the proper orientation. This bond is an extension of the more familiar types. This bond is an extension of the more familiar types. It is a significant aspect of the.
from www.slideserve.com
A quadruple bond is a type of chemical bond between two atoms involving 8 electrons. A quadruple bond is a type of chemical bond between two atoms involving eight electrons. It is a significant aspect of the. A quadruple bond is a type of chemical bond in which four pairs of electrons are shared between two atoms, resulting in a very strong bond. High bonding order possible in main group and may be responsible for the ability to isolate molecular species. There are no arrangements which allow overlap of atomic orbitals that create four bonding molecular orbitals with the proper orientation. This bond is an extension of the more familiar types. A 'quadruple bond' refers to a type of bond between metal atoms where the bond order is greater than 3. C 2 ’s two carbon atoms aren’t joined by a double bond as usually thought, or even. This bond is an extension of the more familiar types.
PPT 2) Evidences for MM double, triple and quadruple bonding
What Is A Quadruple Bond A quadruple bond is a type of chemical bond between two atoms involving 8 electrons. A quadruple bond is a type of chemical bond between two atoms involving 8 electrons. There are no arrangements which allow overlap of atomic orbitals that create four bonding molecular orbitals with the proper orientation. This bond is an extension of the more familiar types. High bonding order possible in main group and may be responsible for the ability to isolate molecular species. A quadruple bond is a type of chemical bond in which four pairs of electrons are shared between two atoms, resulting in a very strong bond. This bond is an extension of the more familiar types. Cx2 and its isoelectronic molecules cnx+, bn and cbx− (each having eight valence electrons) are bound by a quadruple bond. C 2 ’s two carbon atoms aren’t joined by a double bond as usually thought, or even. A quadruple bond is a type of chemical bond between two atoms involving eight electrons. It is a significant aspect of the. A 'quadruple bond' refers to a type of bond between metal atoms where the bond order is greater than 3.
From www.youtube.com
Bonding in [Re2Cl8]2 I QUADRUPLE BOND I ORGANOMETALLIC CHEMISTRY I What Is A Quadruple Bond A quadruple bond is a type of chemical bond in which four pairs of electrons are shared between two atoms, resulting in a very strong bond. Cx2 and its isoelectronic molecules cnx+, bn and cbx− (each having eight valence electrons) are bound by a quadruple bond. A quadruple bond is a type of chemical bond between two atoms involving eight. What Is A Quadruple Bond.
From socratic.org
In the fourth dimension, is it possible to have a quadruple bond What Is A Quadruple Bond There are no arrangements which allow overlap of atomic orbitals that create four bonding molecular orbitals with the proper orientation. A quadruple bond is a type of chemical bond between two atoms involving eight electrons. Cx2 and its isoelectronic molecules cnx+, bn and cbx− (each having eight valence electrons) are bound by a quadruple bond. This bond is an extension. What Is A Quadruple Bond.
From www.youtube.com
Quadruple Bond In Organometallic Chemistry (M.Sc. 2nd ) and CSIR NET What Is A Quadruple Bond C 2 ’s two carbon atoms aren’t joined by a double bond as usually thought, or even. It is a significant aspect of the. There are no arrangements which allow overlap of atomic orbitals that create four bonding molecular orbitals with the proper orientation. Cx2 and its isoelectronic molecules cnx+, bn and cbx− (each having eight valence electrons) are bound. What Is A Quadruple Bond.
From ar.inspiredpencil.com
Quadruple Covalent Bond What Is A Quadruple Bond It is a significant aspect of the. This bond is an extension of the more familiar types. This bond is an extension of the more familiar types. A quadruple bond is a type of chemical bond in which four pairs of electrons are shared between two atoms, resulting in a very strong bond. There are no arrangements which allow overlap. What Is A Quadruple Bond.
From www.ch.ic.ac.uk
Following one's nose a quadruple bond to carbon. Surely I must be What Is A Quadruple Bond This bond is an extension of the more familiar types. A quadruple bond is a type of chemical bond between two atoms involving eight electrons. C 2 ’s two carbon atoms aren’t joined by a double bond as usually thought, or even. A quadruple bond is a type of chemical bond in which four pairs of electrons are shared between. What Is A Quadruple Bond.
From www.slideserve.com
PPT Schedule PowerPoint Presentation, free download ID3220664 What Is A Quadruple Bond High bonding order possible in main group and may be responsible for the ability to isolate molecular species. A quadruple bond is a type of chemical bond between two atoms involving 8 electrons. A quadruple bond is a type of chemical bond between two atoms involving eight electrons. Cx2 and its isoelectronic molecules cnx+, bn and cbx− (each having eight. What Is A Quadruple Bond.
From www.ch.imperial.ac.uk
A new example of a quadruple bond from carbon to Fe. Henry Rzepa's What Is A Quadruple Bond A quadruple bond is a type of chemical bond in which four pairs of electrons are shared between two atoms, resulting in a very strong bond. C 2 ’s two carbon atoms aren’t joined by a double bond as usually thought, or even. Cx2 and its isoelectronic molecules cnx+, bn and cbx− (each having eight valence electrons) are bound by. What Is A Quadruple Bond.
From www.ch.imperial.ac.uk
A new example of a quadruple bond from carbon to Fe. Henry Rzepa's What Is A Quadruple Bond It is a significant aspect of the. A quadruple bond is a type of chemical bond between two atoms involving 8 electrons. A quadruple bond is a type of chemical bond between two atoms involving eight electrons. A quadruple bond is a type of chemical bond in which four pairs of electrons are shared between two atoms, resulting in a. What Is A Quadruple Bond.
From www.ch.imperial.ac.uk
i remind that the nbo method being used to ascertain What Is A Quadruple Bond C 2 ’s two carbon atoms aren’t joined by a double bond as usually thought, or even. Cx2 and its isoelectronic molecules cnx+, bn and cbx− (each having eight valence electrons) are bound by a quadruple bond. There are no arrangements which allow overlap of atomic orbitals that create four bonding molecular orbitals with the proper orientation. A 'quadruple bond'. What Is A Quadruple Bond.
From www.slideserve.com
PPT Schedule PowerPoint Presentation, free download ID3220664 What Is A Quadruple Bond A quadruple bond is a type of chemical bond in which four pairs of electrons are shared between two atoms, resulting in a very strong bond. It is a significant aspect of the. There are no arrangements which allow overlap of atomic orbitals that create four bonding molecular orbitals with the proper orientation. A 'quadruple bond' refers to a type. What Is A Quadruple Bond.
From www.youtube.com
Quadruple Bond I Calculation of Bond Order (Solved Examples) I PartB I What Is A Quadruple Bond A quadruple bond is a type of chemical bond in which four pairs of electrons are shared between two atoms, resulting in a very strong bond. C 2 ’s two carbon atoms aren’t joined by a double bond as usually thought, or even. A quadruple bond is a type of chemical bond between two atoms involving eight electrons. High bonding. What Is A Quadruple Bond.
From www.slideserve.com
PPT 2) Evidences for MM double, triple and quadruple bonding What Is A Quadruple Bond High bonding order possible in main group and may be responsible for the ability to isolate molecular species. Cx2 and its isoelectronic molecules cnx+, bn and cbx− (each having eight valence electrons) are bound by a quadruple bond. A quadruple bond is a type of chemical bond between two atoms involving 8 electrons. This bond is an extension of the. What Is A Quadruple Bond.
From www.mdpi.com
Molecules Free FullText The Role of Quadruple Bonding in the What Is A Quadruple Bond There are no arrangements which allow overlap of atomic orbitals that create four bonding molecular orbitals with the proper orientation. Cx2 and its isoelectronic molecules cnx+, bn and cbx− (each having eight valence electrons) are bound by a quadruple bond. A 'quadruple bond' refers to a type of bond between metal atoms where the bond order is greater than 3.. What Is A Quadruple Bond.
From www.slideserve.com
PPT Multiple Bonds and Hybridization PowerPoint Presentation, free What Is A Quadruple Bond It is a significant aspect of the. This bond is an extension of the more familiar types. A 'quadruple bond' refers to a type of bond between metal atoms where the bond order is greater than 3. This bond is an extension of the more familiar types. A quadruple bond is a type of chemical bond between two atoms involving. What Is A Quadruple Bond.
From www.chemistryworld.com
Theoretical study predicts ironcarbon quadruple bond Research What Is A Quadruple Bond It is a significant aspect of the. A quadruple bond is a type of chemical bond between two atoms involving 8 electrons. A quadruple bond is a type of chemical bond between two atoms involving eight electrons. Cx2 and its isoelectronic molecules cnx+, bn and cbx− (each having eight valence electrons) are bound by a quadruple bond. There are no. What Is A Quadruple Bond.
From alchetron.com
Quadruple bond Alchetron, The Free Social Encyclopedia What Is A Quadruple Bond C 2 ’s two carbon atoms aren’t joined by a double bond as usually thought, or even. High bonding order possible in main group and may be responsible for the ability to isolate molecular species. There are no arrangements which allow overlap of atomic orbitals that create four bonding molecular orbitals with the proper orientation. Cx2 and its isoelectronic molecules. What Is A Quadruple Bond.
From www.chegg.com
Solved l. Certain metal complex dimers contain quadruple What Is A Quadruple Bond High bonding order possible in main group and may be responsible for the ability to isolate molecular species. This bond is an extension of the more familiar types. A quadruple bond is a type of chemical bond in which four pairs of electrons are shared between two atoms, resulting in a very strong bond. This bond is an extension of. What Is A Quadruple Bond.
From www.researchgate.net
duplexes stabilized by quadruple Hbond. Download What Is A Quadruple Bond A 'quadruple bond' refers to a type of bond between metal atoms where the bond order is greater than 3. C 2 ’s two carbon atoms aren’t joined by a double bond as usually thought, or even. It is a significant aspect of the. This bond is an extension of the more familiar types. Cx2 and its isoelectronic molecules cnx+,. What Is A Quadruple Bond.
From www.ch.imperial.ac.uk
A new example of a quadruple bond from carbon to Fe. Henry Rzepa's What Is A Quadruple Bond This bond is an extension of the more familiar types. There are no arrangements which allow overlap of atomic orbitals that create four bonding molecular orbitals with the proper orientation. C 2 ’s two carbon atoms aren’t joined by a double bond as usually thought, or even. Cx2 and its isoelectronic molecules cnx+, bn and cbx− (each having eight valence. What Is A Quadruple Bond.
From www.chegg.com
Solved 1. What is a quadruple bond? How can it be explained What Is A Quadruple Bond This bond is an extension of the more familiar types. A quadruple bond is a type of chemical bond between two atoms involving eight electrons. A quadruple bond is a type of chemical bond in which four pairs of electrons are shared between two atoms, resulting in a very strong bond. A quadruple bond is a type of chemical bond. What Is A Quadruple Bond.
From www.slideserve.com
PPT An Introduction to the Chemistry of Chromium PowerPoint What Is A Quadruple Bond A quadruple bond is a type of chemical bond between two atoms involving 8 electrons. High bonding order possible in main group and may be responsible for the ability to isolate molecular species. This bond is an extension of the more familiar types. A quadruple bond is a type of chemical bond in which four pairs of electrons are shared. What Is A Quadruple Bond.
From www.ch.imperial.ac.uk
A new example of a quadruple bond from carbon to Fe. Henry Rzepa's What Is A Quadruple Bond It is a significant aspect of the. Cx2 and its isoelectronic molecules cnx+, bn and cbx− (each having eight valence electrons) are bound by a quadruple bond. There are no arrangements which allow overlap of atomic orbitals that create four bonding molecular orbitals with the proper orientation. A quadruple bond is a type of chemical bond between two atoms involving. What Is A Quadruple Bond.
From favpng.com
Ditungsten Tetra Quadruple Bond Chemistry Coordination Complex, PNG What Is A Quadruple Bond High bonding order possible in main group and may be responsible for the ability to isolate molecular species. A 'quadruple bond' refers to a type of bond between metal atoms where the bond order is greater than 3. Cx2 and its isoelectronic molecules cnx+, bn and cbx− (each having eight valence electrons) are bound by a quadruple bond. A quadruple. What Is A Quadruple Bond.
From ar.inspiredpencil.com
Quadruple Covalent Bond What Is A Quadruple Bond Cx2 and its isoelectronic molecules cnx+, bn and cbx− (each having eight valence electrons) are bound by a quadruple bond. A quadruple bond is a type of chemical bond in which four pairs of electrons are shared between two atoms, resulting in a very strong bond. C 2 ’s two carbon atoms aren’t joined by a double bond as usually. What Is A Quadruple Bond.
From www.ch.imperial.ac.uk
Blisteringly bent (quadruple) bonds Henry Rzepa's Blog What Is A Quadruple Bond This bond is an extension of the more familiar types. Cx2 and its isoelectronic molecules cnx+, bn and cbx− (each having eight valence electrons) are bound by a quadruple bond. It is a significant aspect of the. A quadruple bond is a type of chemical bond between two atoms involving eight electrons. C 2 ’s two carbon atoms aren’t joined. What Is A Quadruple Bond.
From www.slideshare.net
Unit 11 Covalent Bonding What Is A Quadruple Bond This bond is an extension of the more familiar types. A 'quadruple bond' refers to a type of bond between metal atoms where the bond order is greater than 3. A quadruple bond is a type of chemical bond in which four pairs of electrons are shared between two atoms, resulting in a very strong bond. A quadruple bond is. What Is A Quadruple Bond.
From www.slideserve.com
PPT Schedule PowerPoint Presentation, free download ID3220664 What Is A Quadruple Bond A quadruple bond is a type of chemical bond between two atoms involving eight electrons. Cx2 and its isoelectronic molecules cnx+, bn and cbx− (each having eight valence electrons) are bound by a quadruple bond. High bonding order possible in main group and may be responsible for the ability to isolate molecular species. It is a significant aspect of the.. What Is A Quadruple Bond.
From www.slideserve.com
PPT MetalMetal Bonding PowerPoint Presentation, free download ID What Is A Quadruple Bond High bonding order possible in main group and may be responsible for the ability to isolate molecular species. C 2 ’s two carbon atoms aren’t joined by a double bond as usually thought, or even. Cx2 and its isoelectronic molecules cnx+, bn and cbx− (each having eight valence electrons) are bound by a quadruple bond. A 'quadruple bond' refers to. What Is A Quadruple Bond.
From www.youtube.com
Quadruple bonds [Re2cl8] chemistry organo metallic What Is A Quadruple Bond C 2 ’s two carbon atoms aren’t joined by a double bond as usually thought, or even. A quadruple bond is a type of chemical bond between two atoms involving eight electrons. This bond is an extension of the more familiar types. A quadruple bond is a type of chemical bond in which four pairs of electrons are shared between. What Is A Quadruple Bond.
From www.slideserve.com
PPT Schedule PowerPoint Presentation, free download ID3220664 What Is A Quadruple Bond Cx2 and its isoelectronic molecules cnx+, bn and cbx− (each having eight valence electrons) are bound by a quadruple bond. A quadruple bond is a type of chemical bond between two atoms involving eight electrons. This bond is an extension of the more familiar types. High bonding order possible in main group and may be responsible for the ability to. What Is A Quadruple Bond.
From www.slideshare.net
Unit 11 Covalent Bonding What Is A Quadruple Bond A quadruple bond is a type of chemical bond between two atoms involving 8 electrons. Cx2 and its isoelectronic molecules cnx+, bn and cbx− (each having eight valence electrons) are bound by a quadruple bond. A quadruple bond is a type of chemical bond in which four pairs of electrons are shared between two atoms, resulting in a very strong. What Is A Quadruple Bond.
From www.slideshare.net
Unit 11 Covalent Bonding What Is A Quadruple Bond High bonding order possible in main group and may be responsible for the ability to isolate molecular species. A quadruple bond is a type of chemical bond in which four pairs of electrons are shared between two atoms, resulting in a very strong bond. A quadruple bond is a type of chemical bond between two atoms involving 8 electrons. There. What Is A Quadruple Bond.
From www.youtube.com
quadrupole bonds best analysis 1 csir netjune2018 What Is A Quadruple Bond A 'quadruple bond' refers to a type of bond between metal atoms where the bond order is greater than 3. C 2 ’s two carbon atoms aren’t joined by a double bond as usually thought, or even. This bond is an extension of the more familiar types. A quadruple bond is a type of chemical bond between two atoms involving. What Is A Quadruple Bond.
From www.youtube.com
Quadruple Bond Basic Concept & Structure Organometallic Compound What Is A Quadruple Bond A 'quadruple bond' refers to a type of bond between metal atoms where the bond order is greater than 3. A quadruple bond is a type of chemical bond in which four pairs of electrons are shared between two atoms, resulting in a very strong bond. A quadruple bond is a type of chemical bond between two atoms involving 8. What Is A Quadruple Bond.
From www.researchgate.net
Molecular orbitals involved in the quadruple bond MoMo and their What Is A Quadruple Bond A quadruple bond is a type of chemical bond between two atoms involving 8 electrons. There are no arrangements which allow overlap of atomic orbitals that create four bonding molecular orbitals with the proper orientation. It is a significant aspect of the. A 'quadruple bond' refers to a type of bond between metal atoms where the bond order is greater. What Is A Quadruple Bond.