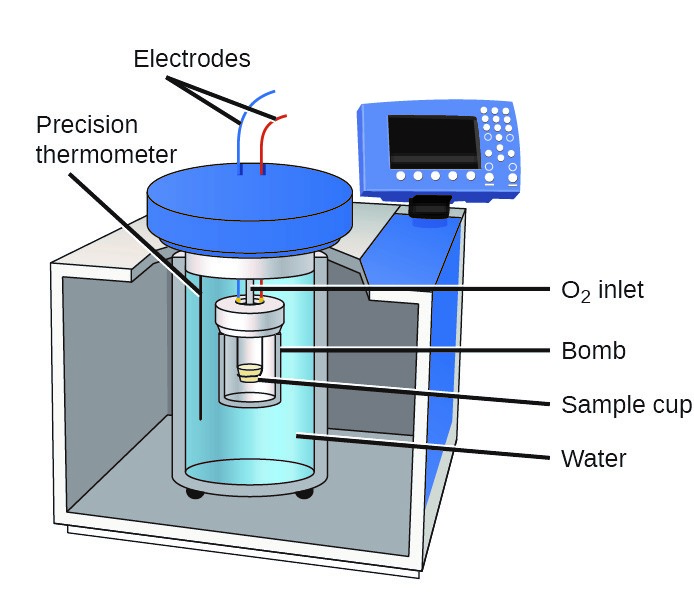
Bomb Calorimeter Wikipedia . Bomb calorimeter an apparatus primarily used for measuring heats of combustion. This lab demonstrates one of the most common. The reaction takes place in a closed space known as the. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Ever wondered exactly how scientists measure the heat content of things? One type in widespread use, called a bomb calorimeter, basically consists of an enclosure in which the reaction takes place, surrounded by a liquid, such as water, that absorbs the heat of the reaction and thus increases in temperature. A tutorial guide on how to calculate the heat of combustion. It might seem like magic, but there’s a clever tool.
from ar.inspiredpencil.com
Bomb calorimeter an apparatus primarily used for measuring heats of combustion. Ever wondered exactly how scientists measure the heat content of things? A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. This lab demonstrates one of the most common. One type in widespread use, called a bomb calorimeter, basically consists of an enclosure in which the reaction takes place, surrounded by a liquid, such as water, that absorbs the heat of the reaction and thus increases in temperature. The reaction takes place in a closed space known as the. A tutorial guide on how to calculate the heat of combustion. It might seem like magic, but there’s a clever tool.
Simple Bomb Calorimeter
Bomb Calorimeter Wikipedia Bomb calorimeter an apparatus primarily used for measuring heats of combustion. A tutorial guide on how to calculate the heat of combustion. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. This lab demonstrates one of the most common. One type in widespread use, called a bomb calorimeter, basically consists of an enclosure in which the reaction takes place, surrounded by a liquid, such as water, that absorbs the heat of the reaction and thus increases in temperature. It might seem like magic, but there’s a clever tool. The reaction takes place in a closed space known as the. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Ever wondered exactly how scientists measure the heat content of things?
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Bomb Calorimeter Wikipedia Ever wondered exactly how scientists measure the heat content of things? The reaction takes place in a closed space known as the. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Bomb calorimeter an apparatus primarily used for measuring heats. Bomb Calorimeter Wikipedia.
From www.thoughtco.com
Calorimeter Definition in Chemistry Bomb Calorimeter Wikipedia The reaction takes place in a closed space known as the. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. This lab demonstrates one of the most common. Ever wondered exactly how scientists measure the heat content of things? A. Bomb Calorimeter Wikipedia.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Bomb Calorimeter Wikipedia A tutorial guide on how to calculate the heat of combustion. The reaction takes place in a closed space known as the. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. Ever wondered exactly how scientists measure the heat content of things? It might seem like magic, but there’s a clever tool. One type in widespread use, called. Bomb Calorimeter Wikipedia.
From people.chem.umass.edu
Untitled Document [people.chem.umass.edu] Bomb Calorimeter Wikipedia It might seem like magic, but there’s a clever tool. This lab demonstrates one of the most common. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. Ever wondered exactly how scientists measure the heat content of things? A tutorial guide on how to calculate the heat of combustion. One type in widespread use, called a bomb calorimeter,. Bomb Calorimeter Wikipedia.
From www.jove.com
Thermochemistry Constant Volume Calorimetry JoVE Book Bomb Calorimeter Wikipedia One type in widespread use, called a bomb calorimeter, basically consists of an enclosure in which the reaction takes place, surrounded by a liquid, such as water, that absorbs the heat of the reaction and thus increases in temperature. Ever wondered exactly how scientists measure the heat content of things? The reaction takes place in a closed space known as. Bomb Calorimeter Wikipedia.
From ar.inspiredpencil.com
Bomb Calorimeter Setup Bomb Calorimeter Wikipedia It might seem like magic, but there’s a clever tool. A tutorial guide on how to calculate the heat of combustion. Ever wondered exactly how scientists measure the heat content of things? The reaction takes place in a closed space known as the. One type in widespread use, called a bomb calorimeter, basically consists of an enclosure in which the. Bomb Calorimeter Wikipedia.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimeter Wikipedia Bomb calorimeter an apparatus primarily used for measuring heats of combustion. It might seem like magic, but there’s a clever tool. Ever wondered exactly how scientists measure the heat content of things? The reaction takes place in a closed space known as the. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned. Bomb Calorimeter Wikipedia.
From engineeringlearn.com
Bomb Calorimeter Definition, Construction, Diagram, Working & Uses Bomb Calorimeter Wikipedia This lab demonstrates one of the most common. Ever wondered exactly how scientists measure the heat content of things? A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. It might seem like magic, but there’s a clever tool. The reaction. Bomb Calorimeter Wikipedia.
From saylordotorg.github.io
Calorimetry Bomb Calorimeter Wikipedia The reaction takes place in a closed space known as the. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. It might seem like magic, but there’s a clever tool. A tutorial guide on how to calculate the heat of. Bomb Calorimeter Wikipedia.
From www.youtube.com
Bomb Calorimetry YouTube Bomb Calorimeter Wikipedia Bomb calorimeter an apparatus primarily used for measuring heats of combustion. The reaction takes place in a closed space known as the. It might seem like magic, but there’s a clever tool. One type in widespread use, called a bomb calorimeter, basically consists of an enclosure in which the reaction takes place, surrounded by a liquid, such as water, that. Bomb Calorimeter Wikipedia.
From foodtechnews.in
What Is Bomb Calorimeter🤔 Measurement of Energy Content in food Food Bomb Calorimeter Wikipedia A tutorial guide on how to calculate the heat of combustion. This lab demonstrates one of the most common. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. It might seem like magic, but there’s a clever tool. Ever wondered exactly how scientists measure the heat content of things? The reaction takes place in a closed space known. Bomb Calorimeter Wikipedia.
From thermonine92.blogspot.com
Thermochemistry Calorimeter Bomb Calorimeter Wikipedia Bomb calorimeter an apparatus primarily used for measuring heats of combustion. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. A tutorial guide on how to calculate the heat of combustion. One type in widespread use, called a bomb calorimeter,. Bomb Calorimeter Wikipedia.
From schoolbag.info
Heat Thermochemistry Training MCAT General Chemistry Review Bomb Calorimeter Wikipedia A tutorial guide on how to calculate the heat of combustion. The reaction takes place in a closed space known as the. It might seem like magic, but there’s a clever tool. Ever wondered exactly how scientists measure the heat content of things? This lab demonstrates one of the most common. One type in widespread use, called a bomb calorimeter,. Bomb Calorimeter Wikipedia.
From www.vrogue.co
What Is Calorimetry With Pictures vrogue.co Bomb Calorimeter Wikipedia A tutorial guide on how to calculate the heat of combustion. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. This lab demonstrates one of the most common. One. Bomb Calorimeter Wikipedia.
From chemlab.truman.edu
Parr 1341 Bomb Calorimeter Chem Lab Bomb Calorimeter Wikipedia Ever wondered exactly how scientists measure the heat content of things? A tutorial guide on how to calculate the heat of combustion. It might seem like magic, but there’s a clever tool. One type in widespread use, called a bomb calorimeter, basically consists of an enclosure in which the reaction takes place, surrounded by a liquid, such as water, that. Bomb Calorimeter Wikipedia.
From scitechdidactic.com
Bomb Calorimeter Model TH 101 Scitech Didactic UK Bomb Calorimeter Wikipedia The reaction takes place in a closed space known as the. It might seem like magic, but there’s a clever tool. A tutorial guide on how to calculate the heat of combustion. This lab demonstrates one of the most common. One type in widespread use, called a bomb calorimeter, basically consists of an enclosure in which the reaction takes place,. Bomb Calorimeter Wikipedia.
From gamma.app
Bomb Calorimeter A Comprehensive Guide Bomb Calorimeter Wikipedia A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. One type in widespread use, called a bomb calorimeter, basically consists of an enclosure in which the reaction takes place,. Bomb Calorimeter Wikipedia.
From commons.wikimedia.org
FileOxygen Bomb Calorimeter.jpg Wikimedia Commons Bomb Calorimeter Wikipedia The reaction takes place in a closed space known as the. A tutorial guide on how to calculate the heat of combustion. This lab demonstrates one of the most common. It might seem like magic, but there’s a clever tool. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen. Bomb Calorimeter Wikipedia.
From www.alamy.com
Bomb calorimeter. This diagram shows the bomb calorimeter designed by Bomb Calorimeter Wikipedia A tutorial guide on how to calculate the heat of combustion. This lab demonstrates one of the most common. It might seem like magic, but there’s a clever tool. Ever wondered exactly how scientists measure the heat content of things? Bomb calorimeter an apparatus primarily used for measuring heats of combustion. The reaction takes place in a closed space known. Bomb Calorimeter Wikipedia.
From martinfersbanks.blogspot.com
Is a Bomb Calorimeter Constant Pressure Bomb Calorimeter Wikipedia The reaction takes place in a closed space known as the. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. Ever wondered exactly how scientists measure the heat content of things? This lab demonstrates one of the most common. A tutorial guide on how to calculate the heat of combustion. It might seem like magic, but there’s a. Bomb Calorimeter Wikipedia.
From www.animalia-life.club
Calorimeter Diagram Bomb Calorimeter Wikipedia A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. One type in widespread use, called a bomb calorimeter, basically consists of an enclosure in which the reaction takes place,. Bomb Calorimeter Wikipedia.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Bomb Calorimeter Wikipedia It might seem like magic, but there’s a clever tool. The reaction takes place in a closed space known as the. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. One type in widespread use, called a bomb calorimeter, basically. Bomb Calorimeter Wikipedia.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Bomb Calorimeter Wikipedia A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. A tutorial guide on how to calculate the heat of combustion. The reaction takes place in a closed space known as the. Bomb calorimeter an apparatus primarily used for measuring heats. Bomb Calorimeter Wikipedia.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimeter Wikipedia A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. It might seem like magic, but there’s a clever tool. The reaction takes place in a closed space known as. Bomb Calorimeter Wikipedia.
From wps.prenhall.com
Media Portfolio Bomb Calorimeter Wikipedia The reaction takes place in a closed space known as the. It might seem like magic, but there’s a clever tool. This lab demonstrates one of the most common. A tutorial guide on how to calculate the heat of combustion. One type in widespread use, called a bomb calorimeter, basically consists of an enclosure in which the reaction takes place,. Bomb Calorimeter Wikipedia.
From courses.lumenlearning.com
Calorimetry Chemistry Bomb Calorimeter Wikipedia This lab demonstrates one of the most common. A tutorial guide on how to calculate the heat of combustion. The reaction takes place in a closed space known as the. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. Ever wondered exactly how scientists measure the heat content of things? One type in widespread use, called a bomb. Bomb Calorimeter Wikipedia.
From www.bartleby.com
Answered A bomb calorimeter, or a constant… bartleby Bomb Calorimeter Wikipedia A tutorial guide on how to calculate the heat of combustion. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. The reaction takes place in a closed space known as the. This lab demonstrates one of the most common. One. Bomb Calorimeter Wikipedia.
From ar.inspiredpencil.com
Simple Bomb Calorimeter Bomb Calorimeter Wikipedia Ever wondered exactly how scientists measure the heat content of things? A tutorial guide on how to calculate the heat of combustion. One type in widespread use, called a bomb calorimeter, basically consists of an enclosure in which the reaction takes place, surrounded by a liquid, such as water, that absorbs the heat of the reaction and thus increases in. Bomb Calorimeter Wikipedia.
From pathwaystochemistry.com
Calorimetry Pathways to Chemistry Bomb Calorimeter Wikipedia It might seem like magic, but there’s a clever tool. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. One type in widespread use, called a bomb calorimeter, basically consists of an enclosure in which the reaction takes place, surrounded. Bomb Calorimeter Wikipedia.
From www.researchgate.net
Schematic sketch of a bomb calorimeter Download Scientific Diagram Bomb Calorimeter Wikipedia This lab demonstrates one of the most common. It might seem like magic, but there’s a clever tool. The reaction takes place in a closed space known as the. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen. Bomb Calorimeter Wikipedia.
From www.animalia-life.club
Calorimeter Diagram Bomb Calorimeter Wikipedia The reaction takes place in a closed space known as the. It might seem like magic, but there’s a clever tool. A tutorial guide on how to calculate the heat of combustion. This lab demonstrates one of the most common. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. Ever wondered exactly how scientists measure the heat content. Bomb Calorimeter Wikipedia.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Bomb Calorimeter Wikipedia Ever wondered exactly how scientists measure the heat content of things? This lab demonstrates one of the most common. It might seem like magic, but there’s a clever tool. One type in widespread use, called a bomb calorimeter, basically consists of an enclosure in which the reaction takes place, surrounded by a liquid, such as water, that absorbs the heat. Bomb Calorimeter Wikipedia.
From byjus.com
What is bomb calorimeter? Bomb Calorimeter Wikipedia A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Ever wondered exactly how scientists measure the heat content of things? This lab demonstrates one of the most common. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. It. Bomb Calorimeter Wikipedia.
From www.youtube.com
Bomb Calorimeter Working and Principle of Bomb Calorimeter GCV of Bomb Calorimeter Wikipedia It might seem like magic, but there’s a clever tool. Bomb calorimeter an apparatus primarily used for measuring heats of combustion. A tutorial guide on how to calculate the heat of combustion. The reaction takes place in a closed space known as the. This lab demonstrates one of the most common. One type in widespread use, called a bomb calorimeter,. Bomb Calorimeter Wikipedia.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Bomb Calorimeter Wikipedia Ever wondered exactly how scientists measure the heat content of things? A tutorial guide on how to calculate the heat of combustion. It might seem like magic, but there’s a clever tool. A bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by. Bomb Calorimeter Wikipedia.