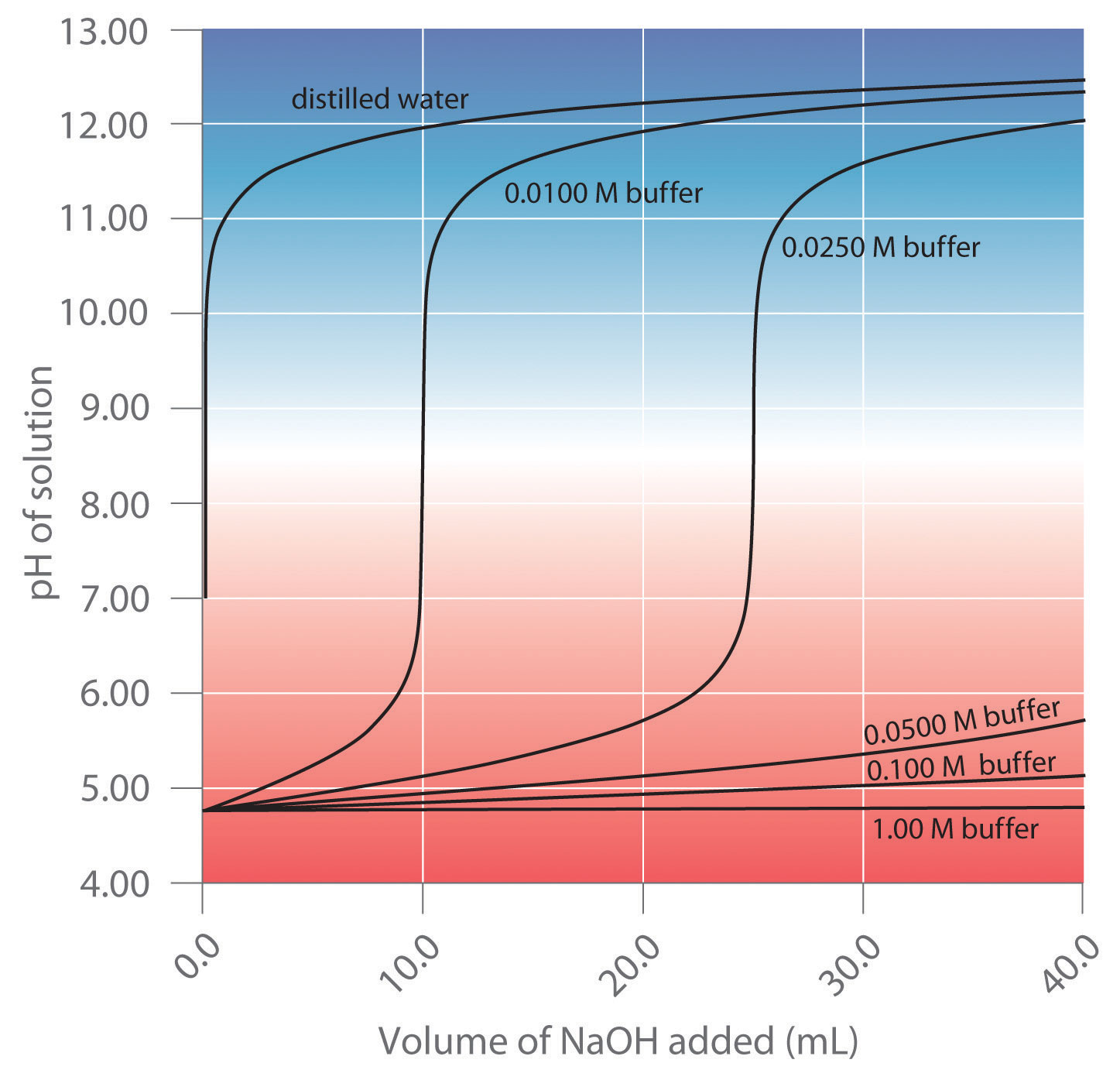
Different Ph Buffers . This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A weak acid and its conjugate base, or b. Buffers maintain ph balance by intercepting acids & bases. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be minimal when a small amount of a strong acid or a strong. Ph buffers and buffer solutions play a crucial role in chemistry, biology and various industries where precise ph control and. A weak base and its conjugate acid. The balanced equation for this reaction is: A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Adding a strong base such as hydroxide would deplete protons from the system, raising.
from saylordotorg.github.io
A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A weak base and its conjugate acid. A weak acid and its conjugate base, or b. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. The balanced equation for this reaction is: Buffers maintain ph balance by intercepting acids & bases. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be minimal when a small amount of a strong acid or a strong. Adding a strong base such as hydroxide would deplete protons from the system, raising. Ph buffers and buffer solutions play a crucial role in chemistry, biology and various industries where precise ph control and. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid.
Buffers
Different Ph Buffers Ph buffers and buffer solutions play a crucial role in chemistry, biology and various industries where precise ph control and. Ph buffers and buffer solutions play a crucial role in chemistry, biology and various industries where precise ph control and. Buffers maintain ph balance by intercepting acids & bases. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. Adding a strong base such as hydroxide would deplete protons from the system, raising. The balanced equation for this reaction is: A weak acid and its conjugate base, or b. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be minimal when a small amount of a strong acid or a strong. A weak base and its conjugate acid. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases.
From labbyag.es
Buffer Ph Range Chart Labb by AG Different Ph Buffers Adding a strong base such as hydroxide would deplete protons from the system, raising. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be minimal when a small amount of a strong acid or a strong. The balanced equation for this reaction is: A. Different Ph Buffers.
From users.highland.edu
Buffers Different Ph Buffers A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be minimal when a small amount of a strong acid or a strong. The balanced equation for this reaction is: Adding a strong base such as hydroxide would deplete protons from the system, raising. Buffers. Different Ph Buffers.
From acidsandbasesfordummieschem.weebly.com
Buffers Acid and Bases for Dummies! Different Ph Buffers Adding a strong base such as hydroxide would deplete protons from the system, raising. The balanced equation for this reaction is: A weak base and its conjugate acid. A weak acid and its conjugate base, or b. Buffers maintain ph balance by intercepting acids & bases. A buffer is a solution that maintains the stability of a system’s ph level. Different Ph Buffers.
From www.instrumentchoice.com.au
pH Buffer Solutions Different Ph Buffers This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Buffers maintain ph balance by intercepting acids & bases. The balanced equation for this reaction is: Ph buffers and buffer solutions play a crucial role in chemistry, biology and various industries where precise ph control and. A buffer is a solution that maintains the stability. Different Ph Buffers.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID Different Ph Buffers Buffers maintain ph balance by intercepting acids & bases. Ph buffers and buffer solutions play a crucial role in chemistry, biology and various industries where precise ph control and. The balanced equation for this reaction is: This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Adding a strong base such as hydroxide would deplete. Different Ph Buffers.
From mmerevise.co.uk
pH Curves Questions and Revision MME Different Ph Buffers The balanced equation for this reaction is: A weak acid and its conjugate base, or b. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer is a solution that maintains the stability. Different Ph Buffers.
From www.slideshare.net
Ph and buffer Different Ph Buffers This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A weak base and its conjugate acid. Ph buffers and buffer solutions play a crucial role in chemistry, biology and various industries where precise ph control and. A buffer solution consists of a weak acid and its conjugate base or a weak base and its. Different Ph Buffers.
From www.youtube.com
CALCULATION pH OF BUFFER SOLUTION EXAMPLE 2 YouTube Different Ph Buffers A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A weak acid and its conjugate base, or b. Buffers maintain ph balance by intercepting acids & bases. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A weak base and its conjugate acid.. Different Ph Buffers.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance Different Ph Buffers A weak base and its conjugate acid. Buffers maintain ph balance by intercepting acids & bases. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change. Different Ph Buffers.
From www.researchgate.net
Solubility of CLT in distilled water, different pH buffers, and 1 w/v Different Ph Buffers A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Adding a strong base such as hydroxide would deplete protons from the system, raising. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change. Different Ph Buffers.
From www.hopaxfc.com
Biological Buffers Products Hopax Fine Chemicals Different Ph Buffers A weak base and its conjugate acid. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be minimal when a small amount of a strong acid or a strong. A buffer solution consists of a weak acid and its conjugate base or a weak. Different Ph Buffers.
From www.awe-ltd.co.uk
pH Buffer Solution for calibrating pH sensors and pH meters Different Ph Buffers A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be minimal when a small amount of a strong acid or a strong.. Different Ph Buffers.
From www.researchgate.net
Dynamics in buffering intensity with pH for different buffers commonly Different Ph Buffers Adding a strong base such as hydroxide would deplete protons from the system, raising. Ph buffers and buffer solutions play a crucial role in chemistry, biology and various industries where precise ph control and. The balanced equation for this reaction is: A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate. Different Ph Buffers.
From www.mcguffmedical.com
pH Calibrating Buffer Solution, pH 4.01, 475mL, Each McGuff Medical Different Ph Buffers Ph buffers and buffer solutions play a crucial role in chemistry, biology and various industries where precise ph control and. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids. Different Ph Buffers.
From www.hamiltoncompany.com
DuraCal pH Buffers Buffer Solution pH Hamilton Company Different Ph Buffers A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A weak base and its conjugate acid. Adding a strong base such as hydroxide would deplete protons from the system, raising. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. The balanced equation for. Different Ph Buffers.
From www.scribd.com
pH and Buffers Acid Buffer Solution Different Ph Buffers The balanced equation for this reaction is: A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Ph buffers and buffer solutions play a crucial role in chemistry, biology and various industries where precise ph. Different Ph Buffers.
From www.shutterstock.com
Bicarbonate Buffer Ph Scale Stock Vector (Royalty Free) 1839896080 Different Ph Buffers A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be minimal when a small amount of a strong acid or a strong. Buffers maintain. Different Ph Buffers.
From www.youtube.com
14.10 Buffers Solutions that Resist pH Change YouTube Different Ph Buffers A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. Ph buffers and buffer solutions play a crucial role in chemistry, biology and various industries where precise ph control and. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids. Different Ph Buffers.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Different Ph Buffers A weak acid and its conjugate base, or b. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be minimal when a small amount of a strong acid or a strong. Buffers maintain ph balance by intercepting acids & bases. A buffer is a. Different Ph Buffers.
From www.amazon.com
pH Buffer Calibration Solution 2Pack pH 4.00 and pH 7.00 — 1 Quart Different Ph Buffers A weak base and its conjugate acid. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Ph buffers and buffer solutions play a crucial role in chemistry, biology and various industries where precise ph control and. A buffer solution consists of a weak acid and its conjugate. Different Ph Buffers.
From giovanna-has-townsend.blogspot.com
What Is the Unique Characterization of a Ph Buffer GiovannahasTownsend Different Ph Buffers Buffers maintain ph balance by intercepting acids & bases. Adding a strong base such as hydroxide would deplete protons from the system, raising. The balanced equation for this reaction is: A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer is a solution that maintains the stability of. Different Ph Buffers.
From www.thoroughflush.co.uk
pH Buffer Solution Pack 4,7 & 10 (65ml) Thoroughflush Different Ph Buffers This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. The balanced equation for this reaction is: A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be minimal when a small amount of a strong acid or a strong.. Different Ph Buffers.
From solutionpharmacy.in
Applications Of Buffers Solution Parmacy Different Ph Buffers This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Buffers maintain ph balance by intercepting acids & bases. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Ph buffers and buffer solutions play a crucial role in chemistry, biology and various. Different Ph Buffers.
From content.byui.edu
Acids, Bases, pH and Buffers Different Ph Buffers A weak acid and its conjugate base, or b. A weak base and its conjugate acid. Buffers maintain ph balance by intercepting acids & bases. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. The balanced equation for this reaction is: A ph buffer is an aqueous solution consisting of a weak acid and. Different Ph Buffers.
From www.dakotainstruments.com
Calibration pH Buffer Pack, 500 ml each of 4.01 pH, 7.00 pH & 10.00 pH Different Ph Buffers A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. Adding a strong base such as hydroxide would deplete protons from the system, raising. A weak acid and its conjugate base, or b. The balanced equation for this reaction is: This characteristic makes buffers important in biological and chemical applications. Different Ph Buffers.
From www.youtube.com
Buffers and pH Biology YouTube Different Ph Buffers A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. The balanced equation for this reaction is: A weak acid and its conjugate base, or b. Adding a strong base such as hydroxide. Different Ph Buffers.
From www.swastikscientificcompany.com
Solution Buffer Ph and Tds Oakton Nist PH Buffer Wholesale Trader Different Ph Buffers A weak acid and its conjugate base, or b. Buffers maintain ph balance by intercepting acids & bases. Ph buffers and buffer solutions play a crucial role in chemistry, biology and various industries where precise ph control and. A weak base and its conjugate acid. A buffer is a solution that maintains the stability of a system’s ph level when. Different Ph Buffers.
From www.lovibond.com
pHBuffer solution Set pH 4, pH 7, pH 10 (25 °C), 90 ml Lovibond Different Ph Buffers This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Adding a strong base such as hydroxide would deplete protons from the system, raising. Buffers maintain ph balance by intercepting acids & bases. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases.. Different Ph Buffers.
From www.researchgate.net
pH of distilled water and pH 7 buffer solution at various temperatures Different Ph Buffers A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be minimal when a small amount of a strong acid or a strong. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A weak base and its conjugate acid.. Different Ph Buffers.
From preclaboratories.com
pH and Buffers How Buffer Solutions Maintain pH Precision Laboratories Different Ph Buffers Buffers maintain ph balance by intercepting acids & bases. The balanced equation for this reaction is: A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be minimal when a small amount of a strong acid or a strong. A weak base and its conjugate. Different Ph Buffers.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Different Ph Buffers A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be minimal when a small amount of a strong acid or a strong. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A buffer solution consists of a weak. Different Ph Buffers.
From saylordotorg.github.io
Buffers Different Ph Buffers This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A weak base and its conjugate acid. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice. Different Ph Buffers.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation, free download ID2948821 Different Ph Buffers The balanced equation for this reaction is: This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be minimal when a small amount of a strong acid or a strong.. Different Ph Buffers.
From www.detect-measure.com
DuraCal pH Buffers Detection & Measurement Systems Different Ph Buffers The balanced equation for this reaction is: A weak base and its conjugate acid. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Adding a strong base such as hydroxide would deplete protons from the system, raising. Ph buffers and buffer solutions play a crucial role in chemistry, biology and various industries where precise. Different Ph Buffers.
From www.walmart.com
Biopharm pH Buffer Calibration Solution Kit 3Pack 250 ml (8oz Different Ph Buffers A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A weak acid and its conjugate base, or b. A weak base and its conjugate acid. Ph buffers and buffer solutions play a crucial role in chemistry, biology and various industries where precise ph control and. The balanced. Different Ph Buffers.