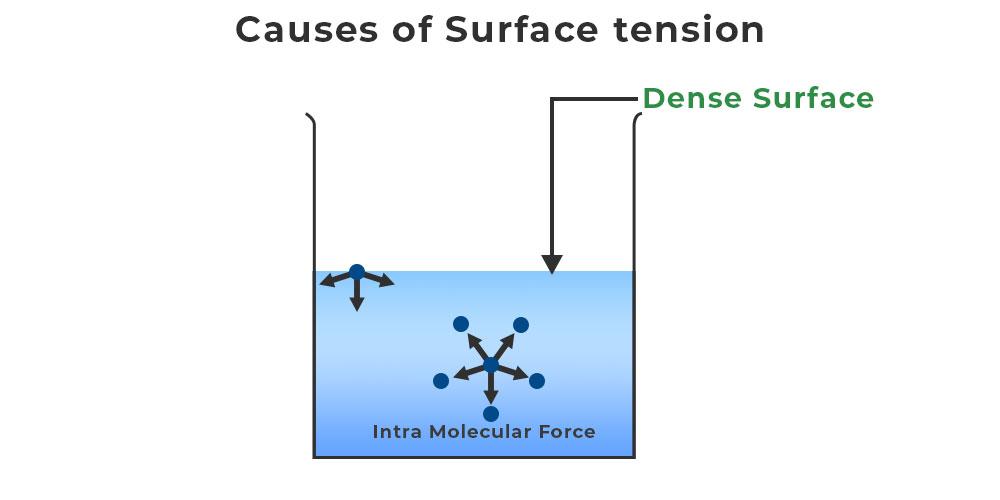
Why Does Surface Tension Exist . Surface tension is a manifestation of the presence of. This phenomenon can be observed in the nearly. Learn the surface tension of water. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surfactants like detergent), each solution exhibits differing surface tension properties. Gasoline) or solutes in the liquid (e.g. This term is typically used only when the liquid surface is in contact with gas (such as the air). Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs many more duties. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Check out a few diagrams. If the surface is between two liquids (such as water and oil), it is called interface tension. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it.
from www.geeksforgeeks.org
Surfactants like detergent), each solution exhibits differing surface tension properties. Surface tension is a manifestation of the presence of. Gasoline) or solutes in the liquid (e.g. Check out a few diagrams. This attraction of molecules towards one another is known as an intermolecular force. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. How does polarity affect it. This term is typically used only when the liquid surface is in contact with gas (such as the air). This phenomenon can be observed in the nearly. Since these intermolecular forces vary depending on the nature of the liquid (e.g.
Surface Tension Definition, Formula, Causes, Examples, and FAQs
Why Does Surface Tension Exist Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. Surfactants like detergent), each solution exhibits differing surface tension properties. Next to mercury, water has the highest surface tension of all commonly occurring liquids. How does polarity affect it. If the surface is between two liquids (such as water and oil), it is called interface tension. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Learn the surface tension of water. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is a manifestation of the presence of. This term is typically used only when the liquid surface is in contact with gas (such as the air). Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs many more duties. This phenomenon can be observed in the nearly. This attraction of molecules towards one another is known as an intermolecular force. Check out a few diagrams.
From civilquery.com
What is Surface Tension Definition, Examples and Tests Civil Query Why Does Surface Tension Exist Check out a few diagrams. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. This attraction of molecules towards one another is known as an intermolecular force. Next to mercury, water has the highest surface tension of all commonly occurring liquids. How does polarity affect it. Surface tension is. Why Does Surface Tension Exist.
From eduinput.com
Surface TensionDefinition, Examples, Causes, And Measurement Why Does Surface Tension Exist Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Learn the surface tension of water. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. Surface tension in water. Why Does Surface Tension Exist.
From courses.lumenlearning.com
Surface Tension Introduction to Chemistry Why Does Surface Tension Exist Surface tension is a manifestation of the presence of. Check out a few diagrams. Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs many more duties. Gasoline) or solutes in the liquid (e.g. Surface tension is a phenomenon in which the surface of. Why Does Surface Tension Exist.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit Why Does Surface Tension Exist This phenomenon can be observed in the nearly. Surface tension is a manifestation of the presence of. Gasoline) or solutes in the liquid (e.g. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Next to mercury, water has the highest surface tension. Why Does Surface Tension Exist.
From www.slideserve.com
PPT Covalent Compounds PowerPoint Presentation, free download ID Why Does Surface Tension Exist Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. This attraction of molecules towards one another is known as an intermolecular force. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.. Why Does Surface Tension Exist.
From www.slideserve.com
PPT Chapter 2 Properties of Fluids PowerPoint Presentation, free Why Does Surface Tension Exist Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane.. Why Does Surface Tension Exist.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector Why Does Surface Tension Exist Learn the surface tension of water. If the surface is between two liquids (such as water and oil), it is called interface tension. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. How does polarity affect it. Surface. Why Does Surface Tension Exist.
From www.slideserve.com
PPT Ch. 2 States of Matter PowerPoint Presentation, free download Why Does Surface Tension Exist Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Learn the. Why Does Surface Tension Exist.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy Why Does Surface Tension Exist Learn the surface tension of water. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. Check out a few diagrams. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Since these intermolecular forces. Why Does Surface Tension Exist.
From www.youtube.com
Surface Tension on the basis of Molecular theory YouTube Why Does Surface Tension Exist Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Next to mercury, water has the highest surface tension of all commonly occurring. Why Does Surface Tension Exist.
From www.slideserve.com
PPT Physical Pharmacy SURFACE TENSION PowerPoint Presentation, free Why Does Surface Tension Exist Surfactants like detergent), each solution exhibits differing surface tension properties. Surface tension is a manifestation of the presence of. Learn the surface tension of water. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Surface tension is the energy, or work, required. Why Does Surface Tension Exist.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog Why Does Surface Tension Exist Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs many more duties. Surface tension is a manifestation of the presence of. Check out a few diagrams. How does polarity affect it. Simply put, surface tension is the tendency of molecules of a liquid. Why Does Surface Tension Exist.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Why Does Surface Tension Exist This attraction of molecules towards one another is known as an intermolecular force. If the surface is between two liquids (such as water and oil), it is called interface tension. This term is typically used only when the liquid surface is in contact with gas (such as the air). Surface tension is a manifestation of the presence of. Simply put,. Why Does Surface Tension Exist.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL Why Does Surface Tension Exist How does polarity affect it. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. If the surface is between two liquids (such as water and oil), it is called interface tension. Surfactants like detergent), each solution exhibits differing surface tension properties. Learn the surface tension of water. Since these. Why Does Surface Tension Exist.
From www.elephango.com
Tension in the Water Educational Resources K12 Learning, Physical Why Does Surface Tension Exist Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Check out a few diagrams. This phenomenon can be observed in the nearly. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension in water might be good at performing tricks, such as being able. Why Does Surface Tension Exist.
From www.merchantnavydecoded.com
What is Surface tension? Why does surface tension occur? Merchant Why Does Surface Tension Exist If the surface is between two liquids (such as water and oil), it is called interface tension. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. Next to mercury, water has the highest surface tension of all commonly. Why Does Surface Tension Exist.
From www.thoughtco.com
What Is Surface Tension? Definition and Experiments Why Does Surface Tension Exist Next to mercury, water has the highest surface tension of all commonly occurring liquids. This term is typically used only when the liquid surface is in contact with gas (such as the air). Learn the surface tension of water. Surface tension is a manifestation of the presence of. Check out a few diagrams. Surface tension, property of a liquid surface. Why Does Surface Tension Exist.
From www.vrogue.co
What Is Surface Tension Molecular Explanation vrogue.co Why Does Surface Tension Exist Check out a few diagrams. Learn the surface tension of water. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Next to mercury, water has the highest surface tension of all commonly occurring liquids. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Surface tension is. Why Does Surface Tension Exist.
From byjus.com
Explain the surface tension phenomenon with examples. Why Does Surface Tension Exist Gasoline) or solutes in the liquid (e.g. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs many more duties. Simply put, surface tension is. Why Does Surface Tension Exist.
From www.slideserve.com
PPT Biomedical importance of surface tension PowerPoint Presentation Why Does Surface Tension Exist Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. This attraction of molecules towards one another is known as an intermolecular force. Learn the surface tension of water. This phenomenon can be observed in the nearly. This term. Why Does Surface Tension Exist.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces Why Does Surface Tension Exist Since these intermolecular forces vary depending on the nature of the liquid (e.g. This phenomenon can be observed in the nearly. Gasoline) or solutes in the liquid (e.g. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Check out a few diagrams.. Why Does Surface Tension Exist.
From www.youtube.com
Surface Tension of Water Explained YouTube Why Does Surface Tension Exist This term is typically used only when the liquid surface is in contact with gas (such as the air). Surfactants like detergent), each solution exhibits differing surface tension properties. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Surface tension is a. Why Does Surface Tension Exist.
From www.youtube.com
The Meaning of Surface Tension and its Practical Applications YouTube Why Does Surface Tension Exist If the surface is between two liquids (such as water and oil), it is called interface tension. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Surfactants like detergent), each solution exhibits differing surface tension properties. Surface tension, property of a liquid. Why Does Surface Tension Exist.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL Why Does Surface Tension Exist This term is typically used only when the liquid surface is in contact with gas (such as the air). Next to mercury, water has the highest surface tension of all commonly occurring liquids. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to. Why Does Surface Tension Exist.
From scienceinfo.com
Surface Tension Definition, Formula, Unit, Causes, Examples, Consequences Why Does Surface Tension Exist Learn the surface tension of water. Since these intermolecular forces vary depending on the nature of the liquid (e.g. This phenomenon can be observed in the nearly. Check out a few diagrams. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the. Why Does Surface Tension Exist.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Why Does Surface Tension Exist Next to mercury, water has the highest surface tension of all commonly occurring liquids. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. Surfactants like detergent), each solution exhibits differing surface tension properties. If the surface is between. Why Does Surface Tension Exist.
From www.expii.com
Surface Tension of Water — Overview & Importance Expii Why Does Surface Tension Exist This phenomenon can be observed in the nearly. How does polarity affect it. Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs many more duties. Gasoline) or solutes in the liquid (e.g. Surface tension, property of a liquid surface displayed by its acting. Why Does Surface Tension Exist.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 Why Does Surface Tension Exist Surface tension is a manifestation of the presence of. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Gasoline) or solutes in. Why Does Surface Tension Exist.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 Why Does Surface Tension Exist Since these intermolecular forces vary depending on the nature of the liquid (e.g. Gasoline) or solutes in the liquid (e.g. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Check out a few diagrams. Learn the surface tension of water. This phenomenon. Why Does Surface Tension Exist.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it Why Does Surface Tension Exist If the surface is between two liquids (such as water and oil), it is called interface tension. How does polarity affect it. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. Surface tension is a phenomenon in which. Why Does Surface Tension Exist.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Why Does Surface Tension Exist Next to mercury, water has the highest surface tension of all commonly occurring liquids. How does polarity affect it. Learn the surface tension of water. Check out a few diagrams. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above. Why Does Surface Tension Exist.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy Why Does Surface Tension Exist Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs many more duties. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Next to mercury, water has the highest surface tension of all commonly occurring liquids. This attraction of molecules. Why Does Surface Tension Exist.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation, free download ID6036532 Why Does Surface Tension Exist Learn the surface tension of water. Next to mercury, water has the highest surface tension of all commonly occurring liquids. This phenomenon can be observed in the nearly. How does polarity affect it. This term is typically used only when the liquid surface is in contact with gas (such as the air). Since these intermolecular forces vary depending on the. Why Does Surface Tension Exist.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Why Does Surface Tension Exist How does polarity affect it. Gasoline) or solutes in the liquid (e.g. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the. Why Does Surface Tension Exist.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Why Does Surface Tension Exist Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. If the surface is between two liquids (such as. Why Does Surface Tension Exist.