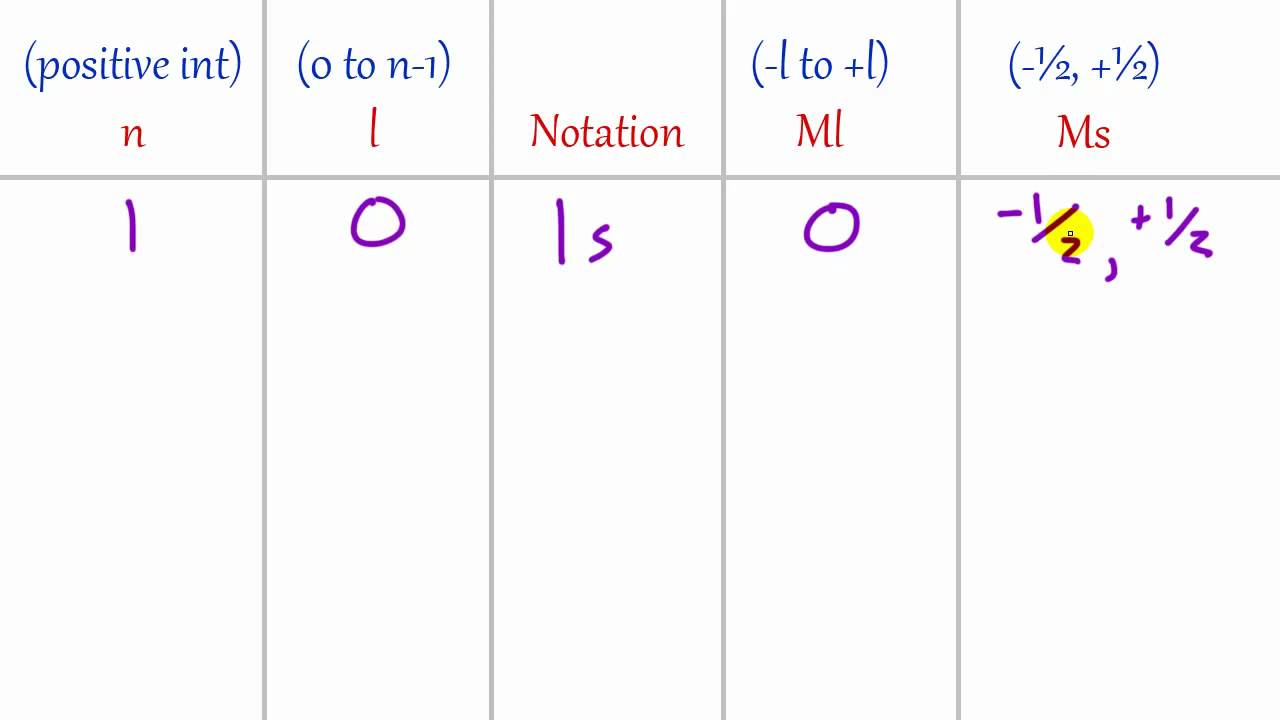
Write Short Note On Quantum Number . Other than principal quantum number (n), spectroscopic notation, magnetic quantum number (m) and the spin quantum number (s), the azimuthal quantum number is another set of quantum numbers which describe the unique quantum state of an electron. Quantum numbers are the values of the preserved. Quantum numbers are divided into four categories: A quantum number is a value that is used when describing the energy levels available to atoms and molecules. Primary, azimuthal, magnetic, and spin. Quantum numbers are numbers assigned to all the electrons in an atom and they describe certain characteristics of the electron. It is very important to understand quantum numbers in order to understand the structure of atom. In this chapter, we’ll learn everything about quantum numbers. Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. An electron in an atom or ion has four quantum numbers to describe its state and. We define \(n\) to be the principal quantum number that labels the basic states of a system. It can be defined as,
from www.youtube.com
It can be defined as, We define \(n\) to be the principal quantum number that labels the basic states of a system. An electron in an atom or ion has four quantum numbers to describe its state and. Other than principal quantum number (n), spectroscopic notation, magnetic quantum number (m) and the spin quantum number (s), the azimuthal quantum number is another set of quantum numbers which describe the unique quantum state of an electron. Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. Quantum numbers are numbers assigned to all the electrons in an atom and they describe certain characteristics of the electron. It is very important to understand quantum numbers in order to understand the structure of atom. Quantum numbers are divided into four categories: Primary, azimuthal, magnetic, and spin. In this chapter, we’ll learn everything about quantum numbers.
Chemistry Lesson 11 Figuring Out the Quantum Numbers YouTube
Write Short Note On Quantum Number Quantum numbers are divided into four categories: In this chapter, we’ll learn everything about quantum numbers. Other than principal quantum number (n), spectroscopic notation, magnetic quantum number (m) and the spin quantum number (s), the azimuthal quantum number is another set of quantum numbers which describe the unique quantum state of an electron. Quantum numbers are the values of the preserved. It can be defined as, Quantum numbers are divided into four categories: Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. A quantum number is a value that is used when describing the energy levels available to atoms and molecules. We define \(n\) to be the principal quantum number that labels the basic states of a system. Quantum numbers are numbers assigned to all the electrons in an atom and they describe certain characteristics of the electron. An electron in an atom or ion has four quantum numbers to describe its state and. It is very important to understand quantum numbers in order to understand the structure of atom. Primary, azimuthal, magnetic, and spin.
From www.priyamstudycentre.com
Quantum Number Orbitals Diagram, Definition, Chart, Shape Write Short Note On Quantum Number In this chapter, we’ll learn everything about quantum numbers. An electron in an atom or ion has four quantum numbers to describe its state and. Quantum numbers are the values of the preserved. Quantum numbers are divided into four categories: We define \(n\) to be the principal quantum number that labels the basic states of a system. A quantum number. Write Short Note On Quantum Number.
From www.numerade.com
SOLVED Activity 4 My Quantum Numbers Identify the quantum numbers Write Short Note On Quantum Number It can be defined as, Quantum numbers are numbers assigned to all the electrons in an atom and they describe certain characteristics of the electron. Quantum numbers are the values of the preserved. Other than principal quantum number (n), spectroscopic notation, magnetic quantum number (m) and the spin quantum number (s), the azimuthal quantum number is another set of quantum. Write Short Note On Quantum Number.
From brainly.in
Determine the values of all the four quantum numbers of the 8th Write Short Note On Quantum Number Other than principal quantum number (n), spectroscopic notation, magnetic quantum number (m) and the spin quantum number (s), the azimuthal quantum number is another set of quantum numbers which describe the unique quantum state of an electron. We define \(n\) to be the principal quantum number that labels the basic states of a system. Quantum numbers are divided into four. Write Short Note On Quantum Number.
From socratic.org
What do the four quantum numbers describe about an electron? Socratic Write Short Note On Quantum Number It is very important to understand quantum numbers in order to understand the structure of atom. A quantum number is a value that is used when describing the energy levels available to atoms and molecules. Primary, azimuthal, magnetic, and spin. Quantum numbers are divided into four categories: Other than principal quantum number (n), spectroscopic notation, magnetic quantum number (m) and. Write Short Note On Quantum Number.
From quantum-lesson.blogspot.com
How To Write Set Of Quantum Numbers Write Short Note On Quantum Number Primary, azimuthal, magnetic, and spin. It is very important to understand quantum numbers in order to understand the structure of atom. Other than principal quantum number (n), spectroscopic notation, magnetic quantum number (m) and the spin quantum number (s), the azimuthal quantum number is another set of quantum numbers which describe the unique quantum state of an electron. We define. Write Short Note On Quantum Number.
From www.youtube.com
Orbitals, Quantum Numbers & Electron Configuration Multiple Choice Write Short Note On Quantum Number We define \(n\) to be the principal quantum number that labels the basic states of a system. It is very important to understand quantum numbers in order to understand the structure of atom. In this chapter, we’ll learn everything about quantum numbers. Quantum numbers are the values of the preserved. Primary, azimuthal, magnetic, and spin. It can be defined as,. Write Short Note On Quantum Number.
From www.numerade.com
SOLVEDAssign a correct set of four quantum numbers for (a) Each Write Short Note On Quantum Number It is very important to understand quantum numbers in order to understand the structure of atom. Quantum numbers are the values of the preserved. An electron in an atom or ion has four quantum numbers to describe its state and. Other than principal quantum number (n), spectroscopic notation, magnetic quantum number (m) and the spin quantum number (s), the azimuthal. Write Short Note On Quantum Number.
From byjus.com
Quantum Numbers (Principal, Azimuthal, and Spin) Definition Write Short Note On Quantum Number Quantum numbers are numbers assigned to all the electrons in an atom and they describe certain characteristics of the electron. It is very important to understand quantum numbers in order to understand the structure of atom. Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron. Write Short Note On Quantum Number.
From www.studypool.com
SOLUTION Quantum mechanics in detail handwritten notes Studypool Write Short Note On Quantum Number An electron in an atom or ion has four quantum numbers to describe its state and. Quantum numbers are the values of the preserved. It can be defined as, Other than principal quantum number (n), spectroscopic notation, magnetic quantum number (m) and the spin quantum number (s), the azimuthal quantum number is another set of quantum numbers which describe the. Write Short Note On Quantum Number.
From eduinput.com
Quantum numbersPrinciple, Azimuthal, and Spin Write Short Note On Quantum Number A quantum number is a value that is used when describing the energy levels available to atoms and molecules. It can be defined as, Other than principal quantum number (n), spectroscopic notation, magnetic quantum number (m) and the spin quantum number (s), the azimuthal quantum number is another set of quantum numbers which describe the unique quantum state of an. Write Short Note On Quantum Number.
From general.chemistrysteps.com
Quantum Numbers Chemistry Steps Write Short Note On Quantum Number Other than principal quantum number (n), spectroscopic notation, magnetic quantum number (m) and the spin quantum number (s), the azimuthal quantum number is another set of quantum numbers which describe the unique quantum state of an electron. Primary, azimuthal, magnetic, and spin. Quantum numbers are numbers assigned to all the electrons in an atom and they describe certain characteristics of. Write Short Note On Quantum Number.
From www.youtube.com
Assigning quantum numbers YouTube Write Short Note On Quantum Number Quantum numbers are numbers assigned to all the electrons in an atom and they describe certain characteristics of the electron. Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. Primary, azimuthal, magnetic, and spin. A quantum number is a value that is used when. Write Short Note On Quantum Number.
From www.doubtnut.com
[Tamil] Write a short note on quantum theory of light. Write Short Note On Quantum Number Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. A quantum number is a value that is used when describing the energy levels available to atoms and molecules. It is very important to understand quantum numbers in order to understand the structure of atom.. Write Short Note On Quantum Number.
From www.docsity.com
Chemistry Planck's Quantum Theory with Example Explaination Write Short Note On Quantum Number An electron in an atom or ion has four quantum numbers to describe its state and. We define \(n\) to be the principal quantum number that labels the basic states of a system. Other than principal quantum number (n), spectroscopic notation, magnetic quantum number (m) and the spin quantum number (s), the azimuthal quantum number is another set of quantum. Write Short Note On Quantum Number.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID6766216 Write Short Note On Quantum Number We define \(n\) to be the principal quantum number that labels the basic states of a system. Quantum numbers are the values of the preserved. It is very important to understand quantum numbers in order to understand the structure of atom. Quantum numbers are divided into four categories: It can be defined as, A quantum number is a value that. Write Short Note On Quantum Number.
From brainly.in
Find the definition Of a quantum number?How many types of quantum Write Short Note On Quantum Number Quantum numbers are divided into four categories: Other than principal quantum number (n), spectroscopic notation, magnetic quantum number (m) and the spin quantum number (s), the azimuthal quantum number is another set of quantum numbers which describe the unique quantum state of an electron. We define \(n\) to be the principal quantum number that labels the basic states of a. Write Short Note On Quantum Number.
From savekhaoyai.blogspot.com
41 quantum number practice worksheet Worksheet Database Write Short Note On Quantum Number It can be defined as, Primary, azimuthal, magnetic, and spin. Other than principal quantum number (n), spectroscopic notation, magnetic quantum number (m) and the spin quantum number (s), the azimuthal quantum number is another set of quantum numbers which describe the unique quantum state of an electron. We define \(n\) to be the principal quantum number that labels the basic. Write Short Note On Quantum Number.
From ar.inspiredpencil.com
Quantum Numbers Write Short Note On Quantum Number Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. Quantum numbers are the values of the preserved. A quantum number is a value that is used when describing the energy levels available to atoms and molecules. In this chapter, we’ll learn everything about quantum. Write Short Note On Quantum Number.
From studylib.net
Lecture 4 Quantum Numbers Write Short Note On Quantum Number We define \(n\) to be the principal quantum number that labels the basic states of a system. An electron in an atom or ion has four quantum numbers to describe its state and. A quantum number is a value that is used when describing the energy levels available to atoms and molecules. In this chapter, we’ll learn everything about quantum. Write Short Note On Quantum Number.
From studylib.net
NOTES ON QUANTUM NUMBERS Write Short Note On Quantum Number A quantum number is a value that is used when describing the energy levels available to atoms and molecules. Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. Other than principal quantum number (n), spectroscopic notation, magnetic quantum number (m) and the spin quantum. Write Short Note On Quantum Number.
From www.studypool.com
SOLUTION Kinematics short notes neet Studypool Write Short Note On Quantum Number A quantum number is a value that is used when describing the energy levels available to atoms and molecules. Other than principal quantum number (n), spectroscopic notation, magnetic quantum number (m) and the spin quantum number (s), the azimuthal quantum number is another set of quantum numbers which describe the unique quantum state of an electron. We define \(n\) to. Write Short Note On Quantum Number.
From www.youtube.com
Chemistry Lesson 11 Figuring Out the Quantum Numbers YouTube Write Short Note On Quantum Number In this chapter, we’ll learn everything about quantum numbers. It is very important to understand quantum numbers in order to understand the structure of atom. Quantum numbers are numbers assigned to all the electrons in an atom and they describe certain characteristics of the electron. Quantum numbers are divided into four categories: Quantum numbers are the values of the preserved.. Write Short Note On Quantum Number.
From www.youtube.com
UPSC Preparation Quantum numbers YouTube Write Short Note On Quantum Number Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. In this chapter, we’ll learn everything about quantum numbers. It is very important to understand quantum numbers in order to understand the structure of atom. Quantum numbers are the values of the preserved. It can. Write Short Note On Quantum Number.
From eduinput.com
Quantum numbersPrinciple, Azimuthal, and Spin Write Short Note On Quantum Number Primary, azimuthal, magnetic, and spin. A quantum number is a value that is used when describing the energy levels available to atoms and molecules. Quantum numbers are the values of the preserved. Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. It can be. Write Short Note On Quantum Number.
From www.youtube.com
How to write quantum numbers in correct way YouTube Write Short Note On Quantum Number Quantum numbers are numbers assigned to all the electrons in an atom and they describe certain characteristics of the electron. It is very important to understand quantum numbers in order to understand the structure of atom. We define \(n\) to be the principal quantum number that labels the basic states of a system. Quantum numbers are the values of the. Write Short Note On Quantum Number.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID3181772 Write Short Note On Quantum Number Quantum numbers are numbers assigned to all the electrons in an atom and they describe certain characteristics of the electron. Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. An electron in an atom or ion has four quantum numbers to describe its state. Write Short Note On Quantum Number.
From www.studypool.com
SOLUTION Quantum numbers worksheet answers Studypool Write Short Note On Quantum Number We define \(n\) to be the principal quantum number that labels the basic states of a system. Other than principal quantum number (n), spectroscopic notation, magnetic quantum number (m) and the spin quantum number (s), the azimuthal quantum number is another set of quantum numbers which describe the unique quantum state of an electron. Quantum numbers are numbers assigned to. Write Short Note On Quantum Number.
From www.pearson.com
Orbitals and Quantum Numbers Pearson+ Channels Write Short Note On Quantum Number Primary, azimuthal, magnetic, and spin. It is very important to understand quantum numbers in order to understand the structure of atom. A quantum number is a value that is used when describing the energy levels available to atoms and molecules. Quantum numbers are divided into four categories: Quantum numbers are the values of the preserved. We define \(n\) to be. Write Short Note On Quantum Number.
From www.quora.com
How to calculate the number of electrons in Mn having a Write Short Note On Quantum Number We define \(n\) to be the principal quantum number that labels the basic states of a system. Quantum numbers are divided into four categories: Other than principal quantum number (n), spectroscopic notation, magnetic quantum number (m) and the spin quantum number (s), the azimuthal quantum number is another set of quantum numbers which describe the unique quantum state of an. Write Short Note On Quantum Number.
From www.chemistrylearner.com
Spin Quantum Number Definition, Significance, and Value Write Short Note On Quantum Number A quantum number is a value that is used when describing the energy levels available to atoms and molecules. Quantum numbers are the values of the preserved. An electron in an atom or ion has four quantum numbers to describe its state and. We define \(n\) to be the principal quantum number that labels the basic states of a system.. Write Short Note On Quantum Number.
From www.freepik.com
Premium Vector Azimuthal quantum number angular measurement physics Write Short Note On Quantum Number In this chapter, we’ll learn everything about quantum numbers. Primary, azimuthal, magnetic, and spin. It is very important to understand quantum numbers in order to understand the structure of atom. Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. A quantum number is a. Write Short Note On Quantum Number.
From www.studypool.com
SOLUTION Note quantum Studypool Write Short Note On Quantum Number Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. In this chapter, we’ll learn everything about quantum numbers. It is very important to understand quantum numbers in order to understand the structure of atom. Other than principal quantum number (n), spectroscopic notation, magnetic quantum. Write Short Note On Quantum Number.
From www.freepik.com
Premium Vector quantum number physics vector illustration Write Short Note On Quantum Number It is very important to understand quantum numbers in order to understand the structure of atom. Quantum numbers are the values of the preserved. Primary, azimuthal, magnetic, and spin. Other than principal quantum number (n), spectroscopic notation, magnetic quantum number (m) and the spin quantum number (s), the azimuthal quantum number is another set of quantum numbers which describe the. Write Short Note On Quantum Number.
From quantum-lesson.blogspot.com
How To Assign Spin Quantum Numbers Write Short Note On Quantum Number Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. Quantum numbers are the values of the preserved. Primary, azimuthal, magnetic, and spin. It can be defined as, Quantum numbers are divided into four categories: An electron in an atom or ion has four quantum. Write Short Note On Quantum Number.
From www.toppr.com
as Is2s 2p. , 2p2p,, Explull. Q. Write note on Principal Quantum Write Short Note On Quantum Number Other than principal quantum number (n), spectroscopic notation, magnetic quantum number (m) and the spin quantum number (s), the azimuthal quantum number is another set of quantum numbers which describe the unique quantum state of an electron. Quantum numbers are divided into four categories: Quantum numbers are a set of numbers used to define the state in which a fundamental. Write Short Note On Quantum Number.