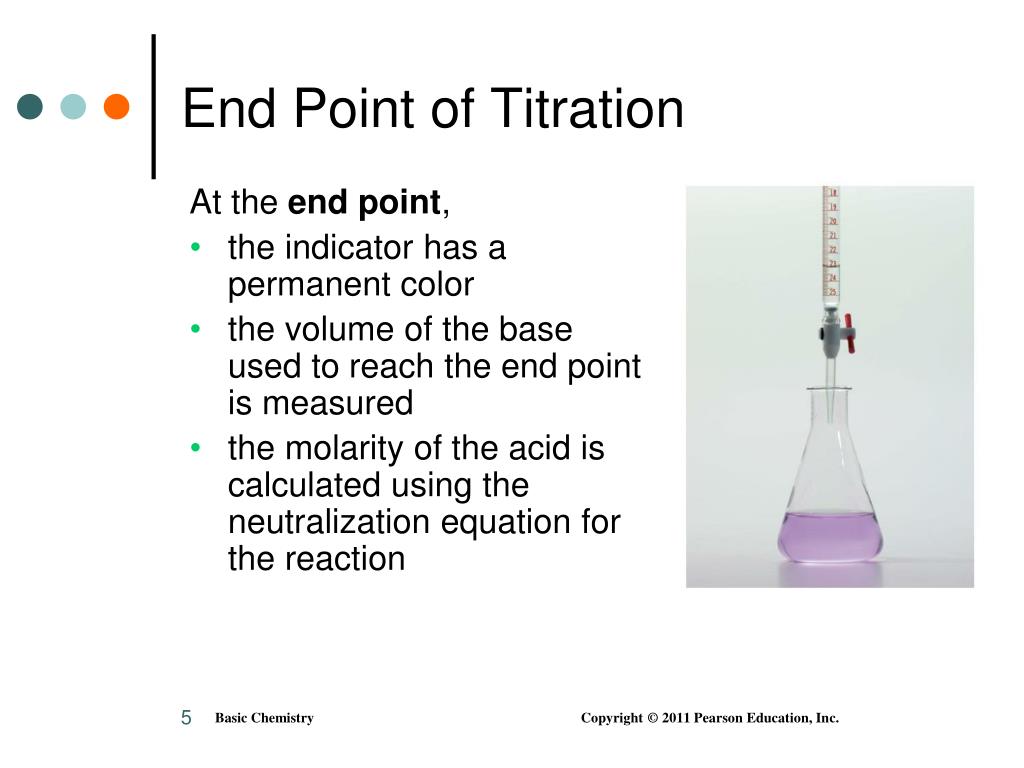
Endpoint Vs Equivalence Point Of Titration . Endpoint and equivalence point are two terms commonly used in titration experiments. Point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution. Equivalence point is the actual point where the chemical reaction in the titration mixture ends. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. the main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction.
from www.slideserve.com
Equivalence point is the actual point where the chemical reaction in the titration mixture ends. Point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution. Endpoint and equivalence point are two terms commonly used in titration experiments. the main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant.
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download
Endpoint Vs Equivalence Point Of Titration Endpoint and equivalence point are two terms commonly used in titration experiments. Point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution. Endpoint and equivalence point are two terms commonly used in titration experiments. Equivalence point is the actual point where the chemical reaction in the titration mixture ends. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. the main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free Endpoint Vs Equivalence Point Of Titration the main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction. Equivalence point is the actual point where the chemical reaction in the titration mixture ends. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant.. Endpoint Vs Equivalence Point Of Titration.
From knowledge-builders.org
What Is The Ph At The Second Equivalence Point Of The Titration Endpoint Vs Equivalence Point Of Titration the main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction. Equivalence point is the actual point where the chemical reaction in the titration mixture ends. Endpoint and equivalence point are two terms commonly used in titration experiments. a titration is a volumetric technique in which a solution of one. Endpoint Vs Equivalence Point Of Titration.
From www.youtube.com
Lecture 7 Titration Half Equivalence Point YouTube Endpoint Vs Equivalence Point Of Titration the main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction. Equivalence point is the actual point where the chemical reaction in the titration mixture ends. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant.. Endpoint Vs Equivalence Point Of Titration.
From www.youtube.com
Difference Between End Point and Equivalence Point YouTube Endpoint Vs Equivalence Point Of Titration a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. the main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction. Equivalence point is the actual point where the chemical reaction in the titration mixture ends.. Endpoint Vs Equivalence Point Of Titration.
From the-equivalent.com
Equivalence point in titration The Equivalent Endpoint Vs Equivalence Point Of Titration Point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. Equivalence point is the actual point where the chemical reaction in the titration mixture ends. . Endpoint Vs Equivalence Point Of Titration.
From chemistry.stackexchange.com
Shape of WeakStrong AcidBase Titration Chemistry Stack Exchange Endpoint Vs Equivalence Point Of Titration a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. Point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution. Endpoint and equivalence point are two terms commonly used in titration experiments. the main difference. Endpoint Vs Equivalence Point Of Titration.
From chem.libretexts.org
11.3 Reaction Stoichiometry in Solutions AcidBase Titrations Endpoint Vs Equivalence Point Of Titration Equivalence point is the actual point where the chemical reaction in the titration mixture ends. the main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction. Point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution. a titration is a volumetric. Endpoint Vs Equivalence Point Of Titration.
From www.youtube.com
Difference between End Point and Equivalence Point Titrations YouTube Endpoint Vs Equivalence Point Of Titration the main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction. Endpoint and equivalence point are two terms commonly used in titration experiments. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. Equivalence point is. Endpoint Vs Equivalence Point Of Titration.
From general.chemistrysteps.com
AcidBase Titrations Chemistry Steps Endpoint Vs Equivalence Point Of Titration a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. Endpoint and equivalence point are two terms commonly used in titration experiments. the main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction. Point in titration. Endpoint Vs Equivalence Point Of Titration.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Endpoint Vs Equivalence Point Of Titration Point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution. Endpoint and equivalence point are two terms commonly used in titration experiments. Equivalence point is the actual point where the chemical reaction in the titration mixture ends. a titration is a volumetric technique in which a solution of one reactant. Endpoint Vs Equivalence Point Of Titration.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Endpoint Vs Equivalence Point Of Titration a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. Equivalence point is the actual point where the chemical reaction in the titration mixture ends. Endpoint and equivalence point are two terms commonly used in titration experiments. the main difference between equivalence and endpoint. Endpoint Vs Equivalence Point Of Titration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Endpoint Vs Equivalence Point Of Titration a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. Point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution. the main difference between equivalence and endpoint is that the equivalence point is a point. Endpoint Vs Equivalence Point Of Titration.
From the-equivalent.com
Find ph at equivalence point The Equivalent Endpoint Vs Equivalence Point Of Titration the main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction. Point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution. Equivalence point is the actual point where the chemical reaction in the titration mixture ends. a titration is a volumetric. Endpoint Vs Equivalence Point Of Titration.
From www.chegg.com
Solved Identify the equivalence point on the titration curve Endpoint Vs Equivalence Point Of Titration Equivalence point is the actual point where the chemical reaction in the titration mixture ends. Endpoint and equivalence point are two terms commonly used in titration experiments. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. the main difference between equivalence and endpoint. Endpoint Vs Equivalence Point Of Titration.
From www.youtube.com
What is the difference between the endpoint and equivalence point in a Endpoint Vs Equivalence Point Of Titration Equivalence point is the actual point where the chemical reaction in the titration mixture ends. Point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution. Endpoint and equivalence point are two terms commonly used in titration experiments. a titration is a volumetric technique in which a solution of one reactant. Endpoint Vs Equivalence Point Of Titration.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Endpoint Vs Equivalence Point Of Titration Equivalence point is the actual point where the chemical reaction in the titration mixture ends. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. Endpoint and equivalence point are two terms commonly used in titration experiments. Point in titration at which the amount of. Endpoint Vs Equivalence Point Of Titration.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Endpoint Vs Equivalence Point Of Titration Point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution. Endpoint and equivalence point are two terms commonly used in titration experiments. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. Equivalence point is the. Endpoint Vs Equivalence Point Of Titration.
From www.differencebetween.com
Difference Between Half Equivalence Point and Equivalence Point Endpoint Vs Equivalence Point Of Titration Endpoint and equivalence point are two terms commonly used in titration experiments. Equivalence point is the actual point where the chemical reaction in the titration mixture ends. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. the main difference between equivalence and endpoint. Endpoint Vs Equivalence Point Of Titration.
From www.numerade.com
SOLVED Explain the difference between end point and equivalence point Endpoint Vs Equivalence Point Of Titration Point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution. Equivalence point is the actual point where the chemical reaction in the titration mixture ends. Endpoint and equivalence point are two terms commonly used in titration experiments. a titration is a volumetric technique in which a solution of one reactant. Endpoint Vs Equivalence Point Of Titration.
From www.chegg.com
How to determine the equivalence point and endpoint Endpoint Vs Equivalence Point Of Titration Endpoint and equivalence point are two terms commonly used in titration experiments. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. the main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction. Equivalence point is. Endpoint Vs Equivalence Point Of Titration.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Endpoint Vs Equivalence Point Of Titration the main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction. Point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution. Equivalence point is the actual point where the chemical reaction in the titration mixture ends. Endpoint and equivalence point are two. Endpoint Vs Equivalence Point Of Titration.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Endpoint Vs Equivalence Point Of Titration Equivalence point is the actual point where the chemical reaction in the titration mixture ends. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. the main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction.. Endpoint Vs Equivalence Point Of Titration.
From edurev.in
End Point And Equivalence Point Mole Concept Chemistry Notes EduRev Endpoint Vs Equivalence Point Of Titration Point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution. Equivalence point is the actual point where the chemical reaction in the titration mixture ends. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. Endpoint. Endpoint Vs Equivalence Point Of Titration.
From www.youtube.com
Titration curves in details. Equivalence point. Half equivalence point Endpoint Vs Equivalence Point Of Titration Equivalence point is the actual point where the chemical reaction in the titration mixture ends. Point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. Endpoint. Endpoint Vs Equivalence Point Of Titration.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download Endpoint Vs Equivalence Point Of Titration Equivalence point is the actual point where the chemical reaction in the titration mixture ends. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. Point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution. . Endpoint Vs Equivalence Point Of Titration.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Endpoint Vs Equivalence Point Of Titration the main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction. Endpoint and equivalence point are two terms commonly used in titration experiments. Equivalence point is the actual point where the chemical reaction in the titration mixture ends. Point in titration at which the amount of titrant added is just enough. Endpoint Vs Equivalence Point Of Titration.
From www.rdchemistry.com
RDChemistry Equivalence Point And End Point Endpoint Vs Equivalence Point Of Titration a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. Endpoint and equivalence point are two terms commonly used in titration experiments. the main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction. Point in titration. Endpoint Vs Equivalence Point Of Titration.
From the-equivalent.com
Equivalence point of titration The Equivalent Endpoint Vs Equivalence Point Of Titration the main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. Endpoint and equivalence point are two terms commonly used in titration experiments. Point in titration. Endpoint Vs Equivalence Point Of Titration.
From www.youtube.com
Titrations Equivalence Point, End Point and Neutralisation Point (A Endpoint Vs Equivalence Point Of Titration a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. the main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction. Point in titration at which the amount of titrant added is just enough to completely. Endpoint Vs Equivalence Point Of Titration.
From www.slideserve.com
PPT CHAPTER 5 Volumetric Analysis PowerPoint Presentation, free Endpoint Vs Equivalence Point Of Titration Equivalence point is the actual point where the chemical reaction in the titration mixture ends. Point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution. the main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction. Endpoint and equivalence point are two. Endpoint Vs Equivalence Point Of Titration.
From romeo-blogmeza.blogspot.com
Explain Equivalence Point and End Point Endpoint Vs Equivalence Point Of Titration Point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution. the main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction. Endpoint and equivalence point are two terms commonly used in titration experiments. Equivalence point is the actual point where the chemical. Endpoint Vs Equivalence Point Of Titration.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Endpoint Vs Equivalence Point Of Titration Equivalence point is the actual point where the chemical reaction in the titration mixture ends. Point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. Endpoint. Endpoint Vs Equivalence Point Of Titration.
From www.animalia-life.club
Endpoint Titration Endpoint Vs Equivalence Point Of Titration a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. the main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction. Equivalence point is the actual point where the chemical reaction in the titration mixture ends.. Endpoint Vs Equivalence Point Of Titration.
From pediaa.com
Difference Between Equivalence Point and Endpoint Definition Endpoint Vs Equivalence Point Of Titration Equivalence point is the actual point where the chemical reaction in the titration mixture ends. Endpoint and equivalence point are two terms commonly used in titration experiments. the main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction. Point in titration at which the amount of titrant added is just enough. Endpoint Vs Equivalence Point Of Titration.
From www.chemistrystudent.com
Finding Ka using a Titration Curve (A2level) ChemistryStudent Endpoint Vs Equivalence Point Of Titration Point in titration at which the amount of titrant added is just enough to completely neutralize the analyte solution. Endpoint and equivalence point are two terms commonly used in titration experiments. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. Equivalence point is the. Endpoint Vs Equivalence Point Of Titration.