Bromine And Chlorine Gas Reaction . When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. The order of reactivity is chlorine > bromine > iodine. Take care to limit students’ exposure to chlorine and bromine water fumes. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. Chlorine with bromides & iodides. Chlorine is also more reactive than the bromine in potassium bromide solution: This is because chlorine could displace bromine and iodine, bromine could only. The colourless solution changes to orange. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. Some students with respiratory problems can show an allergic reaction to chlorine, the onset of which may be delayed. Iodine is the least soluble of the halogens in water. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The violence of the reaction drops considerably as you go from fluorine to chlorine to bromine. At room temperature (20 °c), the physical state of the halogens changes as you go down the group.
from www.chegg.com
The colourless solution changes to orange. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. Chlorine is also more reactive than the bromine in potassium bromide solution: This is because chlorine could displace bromine and iodine, bromine could only. Chlorine with bromides & iodides. At room temperature (20 °c), the physical state of the halogens changes as you go down the group. The violence of the reaction drops considerably as you go from fluorine to chlorine to bromine. The order of reactivity is chlorine > bromine > iodine. Some students with respiratory problems can show an allergic reaction to chlorine, the onset of which may be delayed. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement.
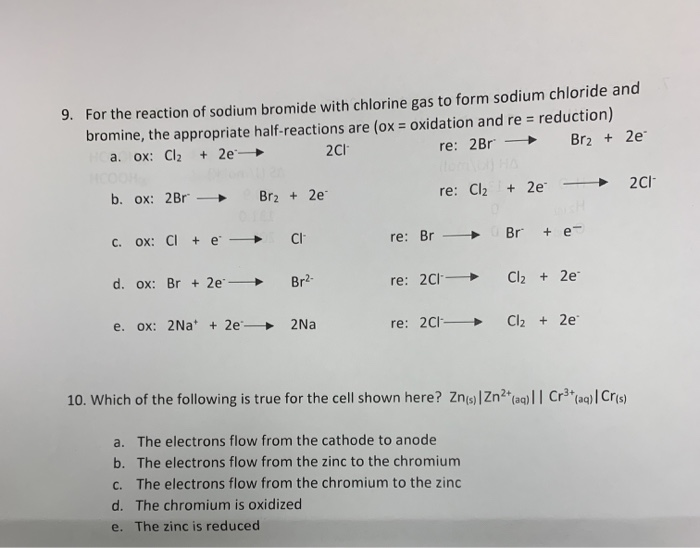
Solved 9. For the reaction of sodium bromide with chlorine
Bromine And Chlorine Gas Reaction Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. The colourless solution changes to orange. The violence of the reaction drops considerably as you go from fluorine to chlorine to bromine. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. Chlorine is also more reactive than the bromine in potassium bromide solution: When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. This is because chlorine could displace bromine and iodine, bromine could only. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Take care to limit students’ exposure to chlorine and bromine water fumes. Some students with respiratory problems can show an allergic reaction to chlorine, the onset of which may be delayed. Chlorine with bromides & iodides. At room temperature (20 °c), the physical state of the halogens changes as you go down the group. The order of reactivity is chlorine > bromine > iodine. Iodine is the least soluble of the halogens in water.
From www.diffzy.com
Bromine vs. Chlorine What's The Difference In Tabular Form, Points Bromine And Chlorine Gas Reaction Some students with respiratory problems can show an allergic reaction to chlorine, the onset of which may be delayed. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. At room temperature (20 °c), the physical state of the halogens changes as you go down the group. When chlorine (as a gas or dissolved in water). Bromine And Chlorine Gas Reaction.
From www.slideserve.com
PPT Chapter Twenty PowerPoint Presentation, free download ID6301153 Bromine And Chlorine Gas Reaction Some students with respiratory problems can show an allergic reaction to chlorine, the onset of which may be delayed. This is because chlorine could displace bromine and iodine, bromine could only. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. Chlorine with bromides & iodides. The order of reactivity is chlorine > bromine > iodine.. Bromine And Chlorine Gas Reaction.
From www.numerade.com
SOLVED According to the following reaction, how many grams of chlorine Bromine And Chlorine Gas Reaction The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. Chlorine is also more reactive than the bromine in potassium bromide solution: Chlorine with bromides & iodides. The colourless solution changes to orange. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an. Bromine And Chlorine Gas Reaction.
From brainly.com
Aluminum bromide and chlorine gas react to form aluminum chloride and Bromine And Chlorine Gas Reaction Chlorine with bromides & iodides. Iodine is the least soluble of the halogens in water. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Some students with respiratory problems can show an allergic reaction to chlorine, the onset of which may be delayed. The reaction between. Bromine And Chlorine Gas Reaction.
From www.numerade.com
SOLVED According to the following reaction, how many grams of chlorine Bromine And Chlorine Gas Reaction The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. At room temperature (20 °c), the physical state of the halogens changes as you go down the group. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. This is because chlorine could displace bromine and iodine, bromine could only. Iodine is. Bromine And Chlorine Gas Reaction.
From www.coursehero.com
[Solved] The reaction of bromine gas with chlorine gas, shown here, has Bromine And Chlorine Gas Reaction The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. At room temperature (20 °c), the physical state. Bromine And Chlorine Gas Reaction.
From studylib.net
Displacement reactions Reactions of chlorine Bromine And Chlorine Gas Reaction Iodine is the least soluble of the halogens in water. The colourless solution changes to orange. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. Some students with respiratory problems can show an allergic reaction to chlorine, the onset of which may be delayed. Chlorine is also more reactive than the bromine in potassium. Bromine And Chlorine Gas Reaction.
From solvedlib.com
45. The reaction of bromine gas with chlorine gas, sh… SolvedLib Bromine And Chlorine Gas Reaction The violence of the reaction drops considerably as you go from fluorine to chlorine to bromine. Take care to limit students’ exposure to chlorine and bromine water fumes. At room temperature (20 °c), the physical state of the halogens changes as you go down the group. The colourless solution changes to orange. If you add chlorine solution to colourless potassium. Bromine And Chlorine Gas Reaction.
From www.youtube.com
Chlorine And Sodium Bromide Make Sodium Chloride And Bromine YouTube Bromine And Chlorine Gas Reaction The colourless solution changes to orange. The violence of the reaction drops considerably as you go from fluorine to chlorine to bromine. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. At room temperature (20 °c), the physical state of the halogens changes as you go down the group. Chlorine is also more reactive than. Bromine And Chlorine Gas Reaction.
From www.alamy.com
Bubbling Chlorine Gas into Sodium Bromide to Yield Bromine Stock Photo Bromine And Chlorine Gas Reaction The colourless solution changes to orange. Chlorine is also more reactive than the bromine in potassium bromide solution: The order of reactivity is chlorine > bromine > iodine. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. The reaction between c=c double bond and bromine (br 2) can be used as a test for the. Bromine And Chlorine Gas Reaction.
From www.sciencephoto.com
Displacement reaction of bromine gas Stock Image A500/0201 Bromine And Chlorine Gas Reaction Chlorine with bromides & iodides. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. Iodine is the least soluble of the halogens in water. The violence of the reaction drops considerably as you go from fluorine to chlorine to bromine. Some students with respiratory problems can show an allergic reaction to chlorine, the onset of. Bromine And Chlorine Gas Reaction.
From www.solutionspile.com
[Solved] 4. At a certain temperature, the equilibrium con Bromine And Chlorine Gas Reaction At room temperature (20 °c), the physical state of the halogens changes as you go down the group. Take care to limit students’ exposure to chlorine and bromine water fumes. Some students with respiratory problems can show an allergic reaction to chlorine, the onset of which may be delayed. The order of reactivity is chlorine > bromine > iodine. The. Bromine And Chlorine Gas Reaction.
From www.youtube.com
NaBr+Cl2=NaCl+Br2 Balanced EquationSodium Bromide+Chlorine=Sodium Bromine And Chlorine Gas Reaction Chlorine is also more reactive than the bromine in potassium bromide solution: The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The violence of the reaction drops considerably as you. Bromine And Chlorine Gas Reaction.
From www.chegg.com
Solved 45. The reaction of bromine gas with chlorine gas, Bromine And Chlorine Gas Reaction The colourless solution changes to orange. Chlorine is also more reactive than the bromine in potassium bromide solution: Take care to limit students’ exposure to chlorine and bromine water fumes. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Iodine is the least soluble. Bromine And Chlorine Gas Reaction.
From www.numerade.com
SOLVED Chlorine gas reacts with aqueous sodium bromide to produce Bromine And Chlorine Gas Reaction This is because chlorine could displace bromine and iodine, bromine could only. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. The order of reactivity is chlorine > bromine > iodine. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. The reaction between c=c double bond and bromine (br 2). Bromine And Chlorine Gas Reaction.
From slideplayer.com
CHEMICAL REACTIONS. ppt download Bromine And Chlorine Gas Reaction Chlorine with bromides & iodides. The colourless solution changes to orange. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. Take care to limit students’ exposure to chlorine and bromine water fumes. The order of reactivity is chlorine > bromine. Bromine And Chlorine Gas Reaction.
From www.coursehero.com
[Solved] 4.7. 3. The reaction of bromine gas with chlorine gas, shown Bromine And Chlorine Gas Reaction Some students with respiratory problems can show an allergic reaction to chlorine, the onset of which may be delayed. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Chlorine with bromides &. Bromine And Chlorine Gas Reaction.
From www.numerade.com
SOLVED Sodium chloride reacts with bromine gas, mathrm{Br}_{2}, to Bromine And Chlorine Gas Reaction Some students with respiratory problems can show an allergic reaction to chlorine, the onset of which may be delayed. This is because chlorine could displace bromine and iodine, bromine could only. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Iodine is the least. Bromine And Chlorine Gas Reaction.
From www.chegg.com
Solved The reaction of bromine gas with chlorine gas Br_2 Bromine And Chlorine Gas Reaction If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. At room temperature (20 °c), the physical state of the halogens changes as you go down the group. The colourless solution changes to orange. Chlorine with bromides & iodides. The order of reactivity is chlorine > bromine > iodine. This is because chlorine could displace. Bromine And Chlorine Gas Reaction.
From solvedlib.com
9/The reaction of bromine gas with chlorine gas, show… SolvedLib Bromine And Chlorine Gas Reaction The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Take care to limit students’ exposure to chlorine and bromine water fumes. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. The order of reactivity is chlorine > bromine >. Bromine And Chlorine Gas Reaction.
From www.numerade.com
SOLVED Aqueous sodium chloride (NaCl) and liquid bromine (Brz are Bromine And Chlorine Gas Reaction The colourless solution changes to orange. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. The violence of the reaction drops considerably as you go from fluorine to chlorine to bromine. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown. Bromine And Chlorine Gas Reaction.
From solvedlib.com
Chlorine and bromine react in the dark with alkenes. … SolvedLib Bromine And Chlorine Gas Reaction Chlorine is also more reactive than the bromine in potassium bromide solution: The violence of the reaction drops considerably as you go from fluorine to chlorine to bromine. This is because chlorine could displace bromine and iodine, bromine could only. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. When chlorine (as a gas or. Bromine And Chlorine Gas Reaction.
From socratic.org
How do you write the equation for this reaction Aluminum bromide and Bromine And Chlorine Gas Reaction The order of reactivity is chlorine > bromine > iodine. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Chlorine with bromides & iodides. Iodine is the least soluble of the halogens in water. Take care to limit students’ exposure to chlorine and bromine water fumes.. Bromine And Chlorine Gas Reaction.
From chem.libretexts.org
10.4 Reactions of Alkenes Addition of Bromine and Chlorine to Alkenes Bromine And Chlorine Gas Reaction The violence of the reaction drops considerably as you go from fluorine to chlorine to bromine. Take care to limit students’ exposure to chlorine and bromine water fumes. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Chlorine with bromides & iodides. The colourless solution changes. Bromine And Chlorine Gas Reaction.
From www.slideserve.com
PPT Q9 What are the chlorine and bromine reactions that destroy Bromine And Chlorine Gas Reaction The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. At room temperature (20 °c), the physical state of the halogens changes as you go down the group. Iodine is the least soluble of the halogens in water. The order of reactivity is chlorine >. Bromine And Chlorine Gas Reaction.
From www.youtube.com
How to Balance NaBr + Cl2 = NaCl + Br2 (Sodium Bromide + Chlorine gas Bromine And Chlorine Gas Reaction At room temperature (20 °c), the physical state of the halogens changes as you go down the group. Chlorine is also more reactive than the bromine in potassium bromide solution: Chlorine with bromides & iodides. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Some students. Bromine And Chlorine Gas Reaction.
From www.numerade.com
SOLVED Q1 State what would be observed, if anything, in the following Bromine And Chlorine Gas Reaction The colourless solution changes to orange. The violence of the reaction drops considerably as you go from fluorine to chlorine to bromine. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. At room temperature (20 °c), the physical state of the halogens changes as you go down the group. This is because chlorine could displace. Bromine And Chlorine Gas Reaction.
From www.numerade.com
SOLVED Aluminum bromide and chlorine gas react to form aluminum Bromine And Chlorine Gas Reaction This is because chlorine could displace bromine and iodine, bromine could only. The order of reactivity is chlorine > bromine > iodine. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. At room temperature (20 °c), the physical state of the. Bromine And Chlorine Gas Reaction.
From www.numerade.com
SOLVED What volume of bromine is required to react with 145 liters of Bromine And Chlorine Gas Reaction The colourless solution changes to orange. This is because chlorine could displace bromine and iodine, bromine could only. Chlorine is also more reactive than the bromine in potassium bromide solution: The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. Take care to limit students’ exposure to chlorine and bromine water fumes. The order of reactivity. Bromine And Chlorine Gas Reaction.
From www.chegg.com
Solved 9. For the reaction of sodium bromide with chlorine Bromine And Chlorine Gas Reaction If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. Chlorine with bromides & iodides. This is because chlorine could displace bromine and iodine, bromine could only. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. At room temperature (20 °c), the physical state of the halogens changes as you. Bromine And Chlorine Gas Reaction.
From slideplayer.com
Chemical Reactions. ppt download Bromine And Chlorine Gas Reaction The order of reactivity is chlorine > bromine > iodine. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. Iodine is the least soluble of the halogens in water. Take care to limit students’ exposure to chlorine and bromine water. Bromine And Chlorine Gas Reaction.
From www.slideshare.net
Oxidation & reduction Bromine And Chlorine Gas Reaction Chlorine with bromides & iodides. The order of reactivity is chlorine > bromine > iodine. Iodine is the least soluble of the halogens in water. Take care to limit students’ exposure to chlorine and bromine water fumes. At room temperature (20 °c), the physical state of the halogens changes as you go down the group. When chlorine (as a gas. Bromine And Chlorine Gas Reaction.
From www.chegg.com
Solved Part A Write a balanced equation for the reaction of Bromine And Chlorine Gas Reaction Chlorine with bromides & iodides. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. Iodine is the least soluble of the halogens in water. The violence of the reaction drops considerably as you go from fluorine to chlorine to bromine. Some students with respiratory problems can show an allergic reaction to chlorine, the onset of. Bromine And Chlorine Gas Reaction.
From www.researchgate.net
Simplified scheme of bromine explosion and ozone destruction reactions Bromine And Chlorine Gas Reaction The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. This is because chlorine could displace bromine and iodine, bromine could only. Chlorine with bromides & iodides. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. The colourless solution changes. Bromine And Chlorine Gas Reaction.
From www.youtube.com
Balancing Chemical Equations. Part 4 Aluminium bromide and chlorine Bromine And Chlorine Gas Reaction The violence of the reaction drops considerably as you go from fluorine to chlorine to bromine. Chlorine is also more reactive than the bromine in potassium bromide solution: At room temperature (20 °c), the physical state of the halogens changes as you go down the group. The reaction between c=c double bond and bromine (br 2) can be used as. Bromine And Chlorine Gas Reaction.