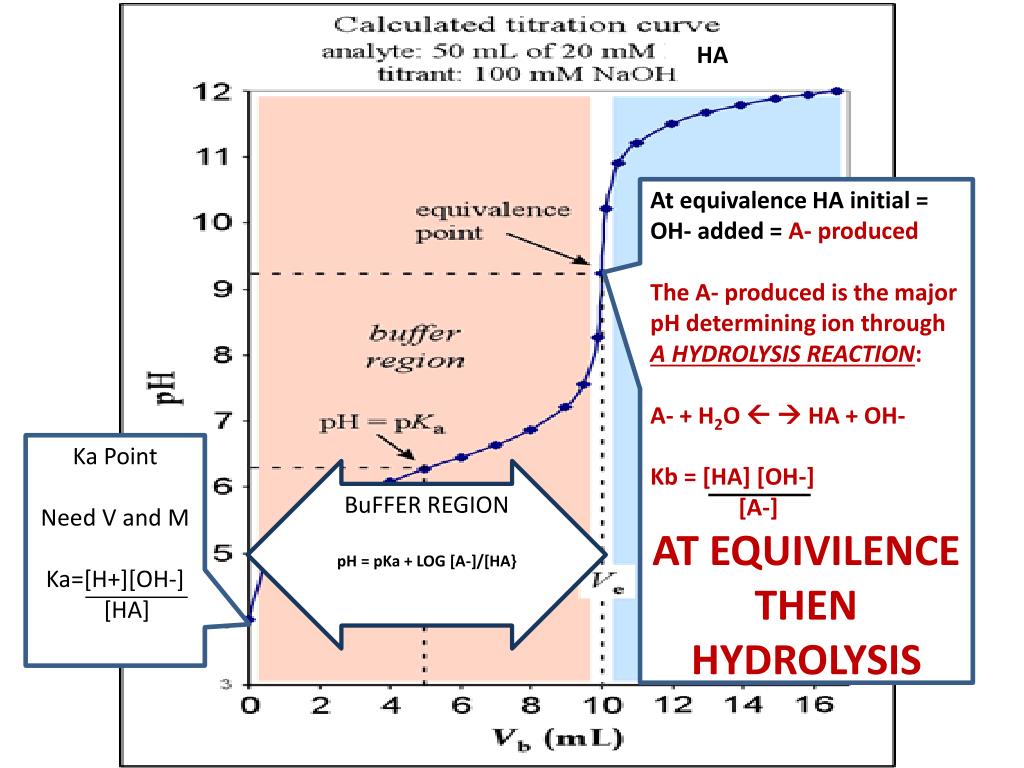
Acid-Base Titration For Equivalence Point . In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a titration curve. The goal is to reach the equivalence point, where the moles of the acid and base are stoichiometrically equal, leading to complete neutralization. In the case of titration of weak acid with strong base, ph at the equivalence point is determined by the weak acid salt hydrolysis. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. The equivalence point of a titration. In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions. In a titration, if the base is added from. Sorting out some confusing terms. Equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or base) that was originally.
from www.slideserve.com
In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a titration curve. The goal is to reach the equivalence point, where the moles of the acid and base are stoichiometrically equal, leading to complete neutralization. In the case of titration of weak acid with strong base, ph at the equivalence point is determined by the weak acid salt hydrolysis. The equivalence point of a titration. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions. Equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or base) that was originally. In a titration, if the base is added from. Sorting out some confusing terms.
PPT TITRATION CURVE WEAK ACID WITH STRONG BASE MGKP 2014 PowerPoint
Acid-Base Titration For Equivalence Point The goal is to reach the equivalence point, where the moles of the acid and base are stoichiometrically equal, leading to complete neutralization. Equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or base) that was originally. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a titration curve. Sorting out some confusing terms. In the case of titration of weak acid with strong base, ph at the equivalence point is determined by the weak acid salt hydrolysis. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. In a titration, if the base is added from. The equivalence point of a titration. The goal is to reach the equivalence point, where the moles of the acid and base are stoichiometrically equal, leading to complete neutralization. In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Acid-Base Titration For Equivalence Point In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a titration curve. In the case of titration of weak acid with strong base, ph at the equivalence point is determined by the weak acid salt hydrolysis. Equivalence (stoichiometric, s) point = theoretical volume at which. Acid-Base Titration For Equivalence Point.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Acid-Base Titration For Equivalence Point The equivalence point of a titration. The goal is to reach the equivalence point, where the moles of the acid and base are stoichiometrically equal, leading to complete neutralization. Equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or base) that was originally. In this section we will learn how. Acid-Base Titration For Equivalence Point.
From www.youtube.com
Weak acid / strong base titration pH after equivalence point YouTube Acid-Base Titration For Equivalence Point Sorting out some confusing terms. The equivalence point of a titration. In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions. The goal is to reach the equivalence point, where the moles of the acid and base are stoichiometrically equal,. Acid-Base Titration For Equivalence Point.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID9681406 Acid-Base Titration For Equivalence Point Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. In the case of titration of weak acid with strong base, ph at the equivalence point is determined by the weak acid salt hydrolysis. In a titration, the equivalence point is the point at. Acid-Base Titration For Equivalence Point.
From schoolbag.info
Titration and Buffers Acids and Bases Training MCAT General Acid-Base Titration For Equivalence Point In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions. In the case of titration of weak acid with strong base, ph at the equivalence point is determined by the weak acid salt hydrolysis. In this section we will learn. Acid-Base Titration For Equivalence Point.
From joiyrusdv.blob.core.windows.net
How To Find Titration Equivalence Point at Douglas Fuller blog Acid-Base Titration For Equivalence Point In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a titration curve. In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions. Equivalence (stoichiometric, s). Acid-Base Titration For Equivalence Point.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Acid-Base Titration For Equivalence Point The equivalence point of a titration. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a titration curve. Sorting out some confusing terms. In the case of titration of weak acid with strong base, ph at the equivalence point is determined by the weak acid. Acid-Base Titration For Equivalence Point.
From www.youtube.com
Weak acid / strong base titration pH at equivalence point YouTube Acid-Base Titration For Equivalence Point In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a titration curve. The goal is to reach the equivalence point, where the moles of the acid and base are stoichiometrically equal, leading to complete neutralization. Sketch out a plot representing the titration of a weak. Acid-Base Titration For Equivalence Point.
From present5.com
AcidBase Titrations Barb Fallon AP Chemistry June 2007 Acid-Base Titration For Equivalence Point Sorting out some confusing terms. Equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or base) that was originally. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. In a titration, the equivalence. Acid-Base Titration For Equivalence Point.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Acid-Base Titration For Equivalence Point Equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or base) that was originally. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. The goal is to reach the equivalence point, where the. Acid-Base Titration For Equivalence Point.
From www.youtube.com
Titration Weak Acid Strong Base Equivalence Point YouTube Acid-Base Titration For Equivalence Point In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a titration curve. Sorting out some. Acid-Base Titration For Equivalence Point.
From dxoeyowlb.blob.core.windows.net
Equivalence Point Base Titration at Myrna Sanchez blog Acid-Base Titration For Equivalence Point Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. The equivalence point of a titration. Sorting out some confusing terms. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to. Acid-Base Titration For Equivalence Point.
From schoolbag.info
What volume of NaOH( aq ) would be needed to reach the equivalence Acid-Base Titration For Equivalence Point Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. In a titration, if the base is added from. The goal is to reach the equivalence point, where the moles of the acid and base are stoichiometrically equal, leading to complete neutralization. In a. Acid-Base Titration For Equivalence Point.
From www.writework.com
Titration of amino acids WriteWork Acid-Base Titration For Equivalence Point Equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or base) that was originally. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. In a titration, the equivalence point is the point at. Acid-Base Titration For Equivalence Point.
From www.youtube.com
Strong acid / strong base titration pH at equivalence point YouTube Acid-Base Titration For Equivalence Point In a titration, if the base is added from. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a titration curve. In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as. Acid-Base Titration For Equivalence Point.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Acid-Base Titration For Equivalence Point Sorting out some confusing terms. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a titration curve. In the case of titration of weak acid with strong base, ph at the equivalence point is determined by the weak acid salt hydrolysis. In a titration, the. Acid-Base Titration For Equivalence Point.
From saylordotorg.github.io
AcidBase Titrations Acid-Base Titration For Equivalence Point The equivalence point of a titration. The goal is to reach the equivalence point, where the moles of the acid and base are stoichiometrically equal, leading to complete neutralization. In a titration, if the base is added from. Sorting out some confusing terms. In the case of titration of weak acid with strong base, ph at the equivalence point is. Acid-Base Titration For Equivalence Point.
From fyoyyrbxr.blob.core.windows.net
Equivalence Point Strong Titration at Darlene Turner blog Acid-Base Titration For Equivalence Point Equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or base) that was originally. In the case of titration of weak acid with strong base, ph at the equivalence point is determined by the weak acid salt hydrolysis. Sorting out some confusing terms. Sketch out a plot representing the titration. Acid-Base Titration For Equivalence Point.
From www.youtube.com
Titration Weak base/strong acid Before the equivalence point YouTube Acid-Base Titration For Equivalence Point In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a titration curve. The goal is to reach the equivalence point, where the moles of the acid and base are stoichiometrically equal, leading to complete neutralization. The equivalence point of a titration. Sketch out a plot. Acid-Base Titration For Equivalence Point.
From dxoqctvwo.blob.core.windows.net
Strong Base Titration Equivalence Point at Dorothy Nicholas blog Acid-Base Titration For Equivalence Point The equivalence point of a titration. The goal is to reach the equivalence point, where the moles of the acid and base are stoichiometrically equal, leading to complete neutralization. In a titration, if the base is added from. Sorting out some confusing terms. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or. Acid-Base Titration For Equivalence Point.
From www.numerade.com
SOLVED Using the following pH curve for the titration of a weak acid Acid-Base Titration For Equivalence Point In the case of titration of weak acid with strong base, ph at the equivalence point is determined by the weak acid salt hydrolysis. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. Equivalence (stoichiometric, s) point = theoretical volume at which moles. Acid-Base Titration For Equivalence Point.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Acid-Base Titration For Equivalence Point The equivalence point of a titration. The goal is to reach the equivalence point, where the moles of the acid and base are stoichiometrically equal, leading to complete neutralization. Sorting out some confusing terms. Equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or base) that was originally. In the. Acid-Base Titration For Equivalence Point.
From goodttorials.blogspot.com
How To Find Equivalence Point From Titration Data Acid-Base Titration For Equivalence Point In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions. In a titration, if the base is added from. The goal is to reach the equivalence point, where the moles of the acid and base are stoichiometrically equal, leading to. Acid-Base Titration For Equivalence Point.
From www.tes.com
Acid Base Titrations, Neutralization, Equivalence Point Grade 11 Acid-Base Titration For Equivalence Point In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a titration curve. In a titration, if the base is added from. Sorting out some confusing terms. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a. Acid-Base Titration For Equivalence Point.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Acid-Base Titration For Equivalence Point The goal is to reach the equivalence point, where the moles of the acid and base are stoichiometrically equal, leading to complete neutralization. In a titration, if the base is added from. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. Equivalence (stoichiometric,. Acid-Base Titration For Equivalence Point.
From www.youtube.com
Acid Base Titration Curves Simplified YouTube Acid-Base Titration For Equivalence Point The equivalence point of a titration. The goal is to reach the equivalence point, where the moles of the acid and base are stoichiometrically equal, leading to complete neutralization. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. In a titration, if the. Acid-Base Titration For Equivalence Point.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Acid-Base Titration For Equivalence Point The equivalence point of a titration. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a titration curve. In the case of titration of weak acid with strong base, ph at the equivalence point is determined by the weak acid salt hydrolysis. In a titration,. Acid-Base Titration For Equivalence Point.
From www.slideserve.com
PPT TITRATION CURVE WEAK ACID WITH STRONG BASE MGKP 2014 PowerPoint Acid-Base Titration For Equivalence Point In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a titration curve. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. In a titration, the equivalence point is. Acid-Base Titration For Equivalence Point.
From mavink.com
Acid Base Titration Curve Acid-Base Titration For Equivalence Point Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. Sorting out some confusing terms. The goal is to reach the equivalence point, where the moles of the acid and base are stoichiometrically equal, leading to complete neutralization. In this section we will learn. Acid-Base Titration For Equivalence Point.
From pressbooks.online.ucf.edu
15.7 AcidBase Titrations Chemistry Fundamentals Acid-Base Titration For Equivalence Point Sorting out some confusing terms. The goal is to reach the equivalence point, where the moles of the acid and base are stoichiometrically equal, leading to complete neutralization. In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions. Equivalence (stoichiometric,. Acid-Base Titration For Equivalence Point.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Acid-Base Titration For Equivalence Point In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles of hydrogen ions. The goal is to reach the equivalence point, where the moles of the acid and base are stoichiometrically equal, leading to complete neutralization. In a titration, if the base is. Acid-Base Titration For Equivalence Point.
From saylordotorg.github.io
AcidBase Titrations Acid-Base Titration For Equivalence Point In the case of titration of weak acid with strong base, ph at the equivalence point is determined by the weak acid salt hydrolysis. In a titration, if the base is added from. In a titration, the equivalence point is the point at which exactly the same number of moles of hydroxide ions have been added as there are moles. Acid-Base Titration For Equivalence Point.
From schoolbag.info
If the acetic acid being titrated here were replaced by hydrochloric Acid-Base Titration For Equivalence Point In the case of titration of weak acid with strong base, ph at the equivalence point is determined by the weak acid salt hydrolysis. The goal is to reach the equivalence point, where the moles of the acid and base are stoichiometrically equal, leading to complete neutralization. Equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or. Acid-Base Titration For Equivalence Point.
From socratic.org
The "pH" at onehalf the equivalence point in an acidbase titration Acid-Base Titration For Equivalence Point In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a titration curve. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. Sorting out some confusing terms. In a. Acid-Base Titration For Equivalence Point.
From dxoqctvwo.blob.core.windows.net
Strong Base Titration Equivalence Point at Dorothy Nicholas blog Acid-Base Titration For Equivalence Point In a titration, if the base is added from. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. In this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. Acid-Base Titration For Equivalence Point.