C In Physics Heat . The specific heat is numerically equal to the. The quantitative relationship between heat transfer and temperature change contains all three factors: The specific heat is the amount of heat necessary to change. C is the specific heat. This formula assumes no phase change occurs during heating. The symbol c stands for specific heat and depends on the material and phase. Heat capacity is the amount of heat required to change the temperature of a certain quantity of the substance by one degree. Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. Δt represents the temperature change in degrees celsius (∘ c) of the substance. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase.
from www.vedantu.com
The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. Δt represents the temperature change in degrees celsius (∘ c) of the substance. Heat capacity is the amount of heat required to change the temperature of a certain quantity of the substance by one degree. The quantitative relationship between heat transfer and temperature change contains all three factors: C is the specific heat. The specific heat is numerically equal to the. This formula assumes no phase change occurs during heating. The symbol c stands for specific heat and depends on the material and phase.
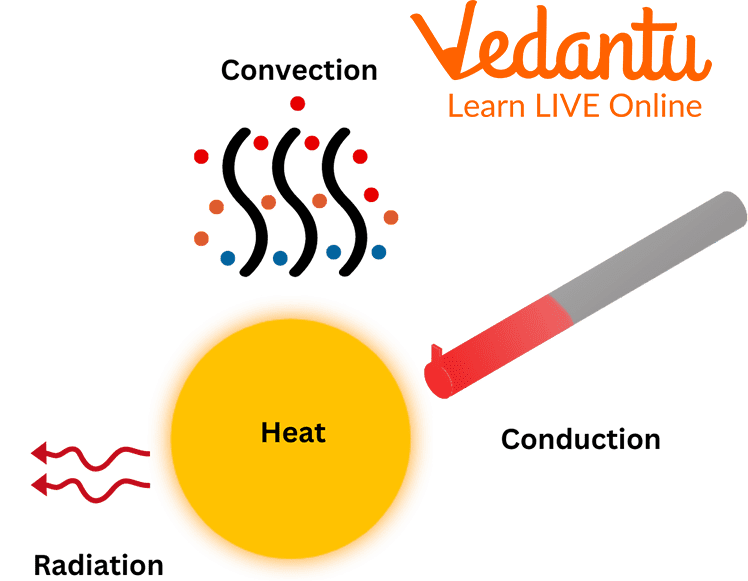
All About of Heat Transfer Overview, Methods, Conduction, Convection
C In Physics Heat Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. The specific heat is the amount of heat necessary to change. C is the specific heat. The symbol c stands for specific heat and depends on the material and phase. The specific heat is numerically equal to the. Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The quantitative relationship between heat transfer and temperature change contains all three factors: Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. This formula assumes no phase change occurs during heating. Heat capacity is the amount of heat required to change the temperature of a certain quantity of the substance by one degree. Δt represents the temperature change in degrees celsius (∘ c) of the substance.
From philschatz.com
The First Law of Thermodynamics and Some Simple Processes · Physics C In Physics Heat C is the specific heat. The specific heat is the amount of heat necessary to change. Heat capacity is the amount of heat required to change the temperature of a certain quantity of the substance by one degree. This formula assumes no phase change occurs during heating. The quantitative relationship between heat transfer and temperature change contains all three factors:. C In Physics Heat.
From quizzlibhofmann.z19.web.core.windows.net
Specific Heat Calculation Examples C In Physics Heat The specific heat is numerically equal to the. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. This formula assumes no phase change occurs during heating. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The quantitative relationship between heat. C In Physics Heat.
From www.dreamstime.com
Heat Transfer Physics Poster, Vector Illustration Diagram with Heat C In Physics Heat Δt represents the temperature change in degrees celsius (∘ c) of the substance. Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. C is the specific heat. The specific heat is numerically equal to the. Q = mcδt, where q is the symbol for heat transfer, m is the mass of. C In Physics Heat.
From me-mechanicalengineering.com
Modes of Heat Transfer C In Physics Heat Heat capacity is the amount of heat required to change the temperature of a certain quantity of the substance by one degree. Δt represents the temperature change in degrees celsius (∘ c) of the substance. C is the specific heat. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase.. C In Physics Heat.
From www.youtube.com
Introduction to Physics Heat and Temperature YouTube C In Physics Heat The specific heat is the amount of heat necessary to change. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The specific heat is numerically equal to the. Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. Δt represents the. C In Physics Heat.
From www.thoughtco.com
Definition and Examples of Heat Energy C In Physics Heat The quantitative relationship between heat transfer and temperature change contains all three factors: Heat capacity is the amount of heat required to change the temperature of a certain quantity of the substance by one degree. The specific heat is numerically equal to the. C is the specific heat. Q = mcδt, where q is the symbol for heat transfer, m. C In Physics Heat.
From www.grc.nasa.gov
Heat Transfer C In Physics Heat Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. Heat capacity is the amount of heat required to change the temperature of a certain quantity of the substance by one degree. Δt represents the temperature change in degrees celsius (∘ c) of the substance. The specific heat is numerically equal to. C In Physics Heat.
From arenahanna.wordpress.com
HEAT WORLD OF PHYSICS steps by steps to understand heat C In Physics Heat The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. The quantitative relationship between heat transfer and temperature change contains all three factors: Δt represents the temperature change in degrees celsius (∘. C In Physics Heat.
From www.tes.com
Effects of Heat (Physics) Teaching Resources C In Physics Heat Heat capacity is the amount of heat required to change the temperature of a certain quantity of the substance by one degree. C is the specific heat. The specific heat is numerically equal to the. The symbol c stands for specific heat and depends on the material and phase. The specific heat is the amount of heat necessary to change.. C In Physics Heat.
From mungfali.com
Heat Transfer Physics C In Physics Heat The quantitative relationship between heat transfer and temperature change contains all three factors: Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. C is the specific heat. The specific heat is. C In Physics Heat.
From www.difference.minaprem.com
Difference Between Heat and Temperature C In Physics Heat Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. Heat capacity is the amount of heat required to change the temperature of a certain quantity of the substance by one degree. The quantitative relationship between heat transfer and temperature change contains all three factors: The specific heat is the amount of. C In Physics Heat.
From mungfali.com
Heat Transfer Physics C In Physics Heat The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The specific heat is the amount of heat necessary to change. The quantitative relationship between heat transfer and temperature change contains all three factors: The symbol c stands for specific heat and depends on the material and phase. The specific. C In Physics Heat.
From www.youtube.com
iGCSE PhysicsThermal Energy Transfer YouTube C In Physics Heat C is the specific heat. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. This formula assumes no phase change occurs during heating. The specific heat is numerically equal to the. The specific heat is the amount of heat necessary to change. The symbol c stands for specific heat. C In Physics Heat.
From www.vedantu.com
All About of Heat Transfer Overview, Methods, Conduction, Convection C In Physics Heat Δt represents the temperature change in degrees celsius (∘ c) of the substance. This formula assumes no phase change occurs during heating. The quantitative relationship between heat transfer and temperature change contains all three factors: C is the specific heat. The specific heat is the amount of heat necessary to change. The specific heat is numerically equal to the. Energy. C In Physics Heat.
From www.doubtnut.com
Principle of heat exchange C In Physics Heat The quantitative relationship between heat transfer and temperature change contains all three factors: The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. This formula assumes no phase change occurs during heating.. C In Physics Heat.
From courses.lumenlearning.com
Temperature Change and Heat Capacity Physics C In Physics Heat Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. Heat capacity is the amount of heat required to change the temperature of a certain quantity of the substance by one degree. Δt represents the temperature change in degrees celsius (∘ c) of the substance. The symbol c stands for specific heat. C In Physics Heat.
From www.youtube.com
Specific Heat Capacity Example Problem Physics YouTube C In Physics Heat Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. The symbol c stands for specific heat and depends on the material and phase. Δt represents the temperature change in degrees celsius (∘ c) of the substance. Heat capacity is the amount of heat required to change the temperature of a certain. C In Physics Heat.
From testbook.com
Heat Energy Definition, Formula, Unit, Examples, Sources, Uses C In Physics Heat Δt represents the temperature change in degrees celsius (∘ c) of the substance. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. The quantitative relationship between heat transfer and temperature change contains all. C In Physics Heat.
From www.youtube.com
Thermodynamics (Physics) Lesson 2 Heat Transfer and Specific Heat.avi C In Physics Heat The symbol c stands for specific heat and depends on the material and phase. This formula assumes no phase change occurs during heating. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The quantitative relationship between heat transfer and temperature change contains all three factors: Δt represents the temperature. C In Physics Heat.
From getrevising.co.uk
Physicsend of year 9 Revision Cards in GCSE Physics C In Physics Heat The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. The quantitative relationship between heat transfer and temperature change contains all three factors: The specific heat is the amount of heat necessary. C In Physics Heat.
From www.youtube.com
What's the Difference between Heat and Temperature? YouTube C In Physics Heat The symbol c stands for specific heat and depends on the material and phase. The specific heat is the amount of heat necessary to change. The specific heat is numerically equal to the. This formula assumes no phase change occurs during heating. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material. C In Physics Heat.
From worksheetfullfunniest.z21.web.core.windows.net
Heat Energy During Phase Change C In Physics Heat Heat capacity is the amount of heat required to change the temperature of a certain quantity of the substance by one degree. Δt represents the temperature change in degrees celsius (∘ c) of the substance. C is the specific heat. The quantitative relationship between heat transfer and temperature change contains all three factors: This formula assumes no phase change occurs. C In Physics Heat.
From www.shutterstock.com
Heat Energy Formula Physics Stock Vector (Royalty Free) 2017214660 C In Physics Heat C is the specific heat. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The specific heat is numerically equal to the. Heat capacity is the amount of heat required to. C In Physics Heat.
From studylib.net
Heat Equation C In Physics Heat Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. The quantitative relationship between heat transfer and temperature change contains all three factors: C is the specific heat. This formula assumes no phase change occurs during heating. The specific heat is the amount of heat necessary to change. The specific heat is. C In Physics Heat.
From www.pinterest.com
Thermal energy physics definition, example with water and C In Physics Heat Heat capacity is the amount of heat required to change the temperature of a certain quantity of the substance by one degree. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Δt represents the temperature change in degrees celsius (∘ c) of the substance. Energy transferred to increase the. C In Physics Heat.
From arenahanna.wordpress.com
HEAT WORLD OF PHYSICS steps by steps to understand heat C In Physics Heat The specific heat is numerically equal to the. Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. C is the specific heat. Δt represents the temperature change in degrees celsius (∘ c) of the substance. Heat capacity is the amount of heat required to change the temperature of a certain quantity. C In Physics Heat.
From examples.yourdictionary.com
Difference Between Heat and Temperature in Simple Terms YourDictionary C In Physics Heat The specific heat is the amount of heat necessary to change. Heat capacity is the amount of heat required to change the temperature of a certain quantity of the substance by one degree. The quantitative relationship between heat transfer and temperature change contains all three factors: This formula assumes no phase change occurs during heating. Δt represents the temperature change. C In Physics Heat.
From www.youtube.com
Specific Heat Capacity Mr E Physics P3 AQA GCSE Physics YouTube C In Physics Heat Heat capacity is the amount of heat required to change the temperature of a certain quantity of the substance by one degree. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. The symbol. C In Physics Heat.
From www.ces.fau.edu
Climate Science Investigations South Florida Energy The Driver of C In Physics Heat C is the specific heat. The specific heat is numerically equal to the. Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. Heat capacity is the amount of heat required to change the temperature of a certain quantity of the substance by one degree. The specific heat is the amount of. C In Physics Heat.
From leverageedu.com
Thermal Properties of Matter Class 11 Physics Notes Leverage Edu C In Physics Heat This formula assumes no phase change occurs during heating. The quantitative relationship between heat transfer and temperature change contains all three factors: The symbol c stands for specific heat and depends on the material and phase. The specific heat is the amount of heat necessary to change. The symbol c stands for the specific heat (also called “specific heat capacity”). C In Physics Heat.
From sciencenotes.org
What Is Temperature? Definition in Science C In Physics Heat The specific heat is numerically equal to the. Heat capacity is the amount of heat required to change the temperature of a certain quantity of the substance by one degree. Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. The specific heat is the amount of heat necessary to change. C. C In Physics Heat.
From www.tes.com
GCSE Physics Specific Heat Capacity Teaching Resources C In Physics Heat This formula assumes no phase change occurs during heating. The quantitative relationship between heat transfer and temperature change contains all three factors: Q = mcδt, where q is the symbol for heat transfer, m is the mass of the substance,. Energy transferred to increase the temperature of a substance by heating is proportional to its specific heat capacity. Δt represents. C In Physics Heat.
From stock.adobe.com
Heat energy as convection, conduction and radiation, physics science C In Physics Heat This formula assumes no phase change occurs during heating. The quantitative relationship between heat transfer and temperature change contains all three factors: The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Δt represents the temperature change in degrees celsius (∘ c) of the substance. The specific heat is the. C In Physics Heat.
From ffden-2.phys.uaf.edu
Thermodynamics C In Physics Heat Δt represents the temperature change in degrees celsius (∘ c) of the substance. The quantitative relationship between heat transfer and temperature change contains all three factors: The specific heat is the amount of heat necessary to change. This formula assumes no phase change occurs during heating. C is the specific heat. Energy transferred to increase the temperature of a substance. C In Physics Heat.
From animalia-life.club
Examples Of Heat Energy C In Physics Heat The quantitative relationship between heat transfer and temperature change contains all three factors: The symbol c stands for specific heat and depends on the material and phase. Δt represents the temperature change in degrees celsius (∘ c) of the substance. C is the specific heat. The specific heat is numerically equal to the. This formula assumes no phase change occurs. C In Physics Heat.