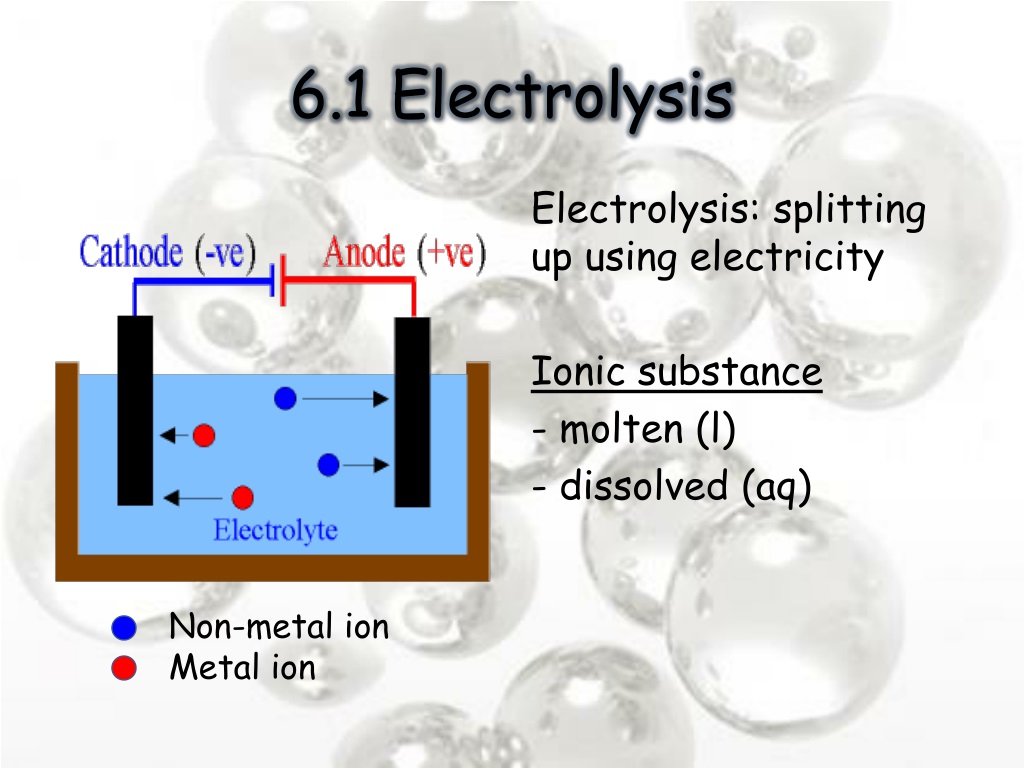
Negative Electrode In The Electrolysis . Na + ions combine with the free. When they get there, 2. Learn how to predict the products of electrolysis of different. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. Find out the definitions and examples of anode and. The negative electrode during electrolysis is called the cathode. Learn about electrolysis, the decomposition of a compound using an electric current. Positive lead(ii) ions are attracted to it. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. The cathode (the negative electrode) has an abundance of electrons being pumped on to it by the power source. Cation is a positively charged ion which is attracted to the cathode. Cathode is the negative electrode of an electrolysis cell. Electrolysis is a process in which an external circuit does work on a redox system to drive a nonspontaneous reaction. Learn about electrolytic cells, which are devices that use electrical energy to drive nonspontaneous redox reactions.
from www.slideserve.com
Learn about electrolytic cells, which are devices that use electrical energy to drive nonspontaneous redox reactions. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. Cation is a positively charged ion which is attracted to the cathode. Learn about electrolysis, the decomposition of a compound using an electric current. The cathode (the negative electrode) has an abundance of electrons being pumped on to it by the power source. Electrolysis is a process in which an external circuit does work on a redox system to drive a nonspontaneous reaction. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. Cathode is the negative electrode of an electrolysis cell. Positive lead(ii) ions are attracted to it. When they get there, 2.
PPT Electrolysis PowerPoint Presentation, free download ID9353258
Negative Electrode In The Electrolysis Find out the definitions and examples of anode and. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. Na + ions combine with the free. Cation is a positively charged ion which is attracted to the cathode. Learn about electrolytic cells, which are devices that use electrical energy to drive nonspontaneous redox reactions. Learn how to predict the products of electrolysis of different. Learn about electrolysis, the decomposition of a compound using an electric current. The cathode (the negative electrode) has an abundance of electrons being pumped on to it by the power source. Electrolysis is a process in which an external circuit does work on a redox system to drive a nonspontaneous reaction. Find out the definitions and examples of anode and. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. The negative electrode during electrolysis is called the cathode. When they get there, 2. Cathode is the negative electrode of an electrolysis cell. Positive lead(ii) ions are attracted to it.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace Negative Electrode In The Electrolysis Cation is a positively charged ion which is attracted to the cathode. Positive lead(ii) ions are attracted to it. The negative electrode during electrolysis is called the cathode. The cathode (the negative electrode) has an abundance of electrons being pumped on to it by the power source. Na + ions combine with the free. When they get there, 2. Learn. Negative Electrode In The Electrolysis.
From enginelibsaprozoic.z21.web.core.windows.net
What Happens At The Cathode In Electrolysis Negative Electrode In The Electrolysis Cation is a positively charged ion which is attracted to the cathode. Find out the definitions and examples of anode and. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. Na + ions combine with the free. When they get there, 2. Learn about electrolysis, the decomposition of a compound using an. Negative Electrode In The Electrolysis.
From general.chemistrysteps.com
Electrolysis Chemistry Steps Negative Electrode In The Electrolysis Electrolysis is a process in which an external circuit does work on a redox system to drive a nonspontaneous reaction. Positive lead(ii) ions are attracted to it. Learn how to predict the products of electrolysis of different. When they get there, 2. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions. Negative Electrode In The Electrolysis.
From shop.wf-education.com
Understanding Electrolysis Negative Electrode In The Electrolysis The negative electrode during electrolysis is called the cathode. Learn about electrolytic cells, which are devices that use electrical energy to drive nonspontaneous redox reactions. Positive lead(ii) ions are attracted to it. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. Find out the definitions and examples of anode and. The cathode. Negative Electrode In The Electrolysis.
From byjus.com
What is the negative electrode called in electrolysis? Negative Electrode In The Electrolysis Cation is a positively charged ion which is attracted to the cathode. When they get there, 2. Learn about electrolytic cells, which are devices that use electrical energy to drive nonspontaneous redox reactions. Na + ions combine with the free. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the. Negative Electrode In The Electrolysis.
From vdocuments.mx
negative electrode (anode) positive electrode (cathode ) anode cathode Negative Electrode In The Electrolysis Cathode is the negative electrode of an electrolysis cell. The negative electrode during electrolysis is called the cathode. Positive lead(ii) ions are attracted to it. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. Learn about electrolytic cells, which are devices that use electrical energy to drive nonspontaneous. Negative Electrode In The Electrolysis.
From www.slideserve.com
PPT Electrolysis of Solutions PowerPoint Presentation, free download Negative Electrode In The Electrolysis The cathode (the negative electrode) has an abundance of electrons being pumped on to it by the power source. Learn about electrolysis, the decomposition of a compound using an electric current. Cation is a positively charged ion which is attracted to the cathode. Find out the definitions and examples of anode and. Na + ions combine with the free. Learn. Negative Electrode In The Electrolysis.
From www.elevise.co.uk
C4 K) Electrolysis Part 2 AQA Combined Science Trilogy Elevise Negative Electrode In The Electrolysis The negative electrode during electrolysis is called the cathode. The cathode (the negative electrode) has an abundance of electrons being pumped on to it by the power source. Find out the definitions and examples of anode and. Electrolysis is a process in which an external circuit does work on a redox system to drive a nonspontaneous reaction. Na + ions. Negative Electrode In The Electrolysis.
From brilliant.org
Electrolytic Cells and Electrolysis Brilliant Math & Science Wiki Negative Electrode In The Electrolysis Learn how to predict the products of electrolysis of different. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. The cathode (the negative electrode) has an abundance of electrons being pumped on to it by the power source. Cathode is the negative electrode of an electrolysis cell. The. Negative Electrode In The Electrolysis.
From byjus.com
In electrolysis what are the positive and negative electrodes known as?? Negative Electrode In The Electrolysis Learn how to predict the products of electrolysis of different. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. Electrolysis is a process in which an external circuit does work on a redox system to drive a nonspontaneous reaction. The cathode (the negative electrode) has an abundance of electrons being pumped on. Negative Electrode In The Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID9353258 Negative Electrode In The Electrolysis Na + ions combine with the free. Learn about electrolytic cells, which are devices that use electrical energy to drive nonspontaneous redox reactions. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. The negative electrode during electrolysis is called the cathode. Cation is a positively charged ion which is attracted to the. Negative Electrode In The Electrolysis.
From slidetodoc.com
Electrolysis background Electrodes Conducting liquid electrolyte Negative Electrode In The Electrolysis Cation is a positively charged ion which is attracted to the cathode. When they get there, 2. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. The cathode (the negative electrode) has an abundance of electrons being pumped on to it by the power source. Learn about electrolysis, the decomposition of a. Negative Electrode In The Electrolysis.
From webmis.highland.cc.il.us
Electrolysis Negative Electrode In The Electrolysis Cathode is the negative electrode of an electrolysis cell. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. When they get there, 2. The negative electrode during electrolysis is called the cathode. Learn about electrolytic cells, which are devices that use electrical energy to drive nonspontaneous redox reactions. Learn about electrolysis, the. Negative Electrode In The Electrolysis.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.58C Describe Experiments to Investigate Negative Electrode In The Electrolysis Learn about electrolytic cells, which are devices that use electrical energy to drive nonspontaneous redox reactions. When they get there, 2. Learn about electrolysis, the decomposition of a compound using an electric current. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. Electrolysis is a process in which. Negative Electrode In The Electrolysis.
From byjus.com
An electrolytic cell is used to convert Negative Electrode In The Electrolysis When they get there, 2. The cathode (the negative electrode) has an abundance of electrons being pumped on to it by the power source. Electrolysis is a process in which an external circuit does work on a redox system to drive a nonspontaneous reaction. Find out the definitions and examples of anode and. Learn about electrolysis, the decomposition of a. Negative Electrode In The Electrolysis.
From stock.adobe.com
Vector scientific illustration of the electrolysis processes. Set of Negative Electrode In The Electrolysis Find out the definitions and examples of anode and. Cation is a positively charged ion which is attracted to the cathode. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. When they get there, 2. The negative electrode during electrolysis is called the cathode. Learn how to predict the products of electrolysis. Negative Electrode In The Electrolysis.
From www.elevise.co.uk
C4 K) Electrolysis Part 2 AQA Combined Science Trilogy Elevise Negative Electrode In The Electrolysis Cation is a positively charged ion which is attracted to the cathode. Positive lead(ii) ions are attracted to it. Learn how to predict the products of electrolysis of different. When they get there, 2. Find out the definitions and examples of anode and. Na + ions combine with the free. The electrode attached to the negative terminal of a battery. Negative Electrode In The Electrolysis.
From www.thoughtco.com
How to Define Anode and Cathode Negative Electrode In The Electrolysis Cation is a positively charged ion which is attracted to the cathode. Find out the definitions and examples of anode and. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. Na. Negative Electrode In The Electrolysis.
From www.dreamstime.com
Electrolysis of Water Splitting H2O Molecules Stock Illustration Negative Electrode In The Electrolysis Na + ions combine with the free. When they get there, 2. Positive lead(ii) ions are attracted to it. Learn about electrolytic cells, which are devices that use electrical energy to drive nonspontaneous redox reactions. The negative electrode during electrolysis is called the cathode. The electrode attached to the negative terminal of a battery is called a negative electrode, or. Negative Electrode In The Electrolysis.
From www.slideserve.com
PPT Electrolysis L.O. I know and can use the terms electrolyte Negative Electrode In The Electrolysis Learn about electrolysis, the decomposition of a compound using an electric current. Electrolysis is a process in which an external circuit does work on a redox system to drive a nonspontaneous reaction. When they get there, 2. Learn about electrolytic cells, which are devices that use electrical energy to drive nonspontaneous redox reactions. Cation is a positively charged ion which. Negative Electrode In The Electrolysis.
From www.slideserve.com
PPT 17.78 Electrolysis & Applications PowerPoint Presentation ID Negative Electrode In The Electrolysis When they get there, 2. Learn how to predict the products of electrolysis of different. Learn about electrolysis, the decomposition of a compound using an electric current. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na. Negative Electrode In The Electrolysis.
From www.alamy.com
Electrolysis hires stock photography and images Alamy Negative Electrode In The Electrolysis Find out the definitions and examples of anode and. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. Electrolysis is a process in which an external circuit does work on a redox system to drive a nonspontaneous reaction. The cathode (the negative electrode) has an abundance of electrons being pumped on to. Negative Electrode In The Electrolysis.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID6499367 Negative Electrode In The Electrolysis Cathode is the negative electrode of an electrolysis cell. The cathode (the negative electrode) has an abundance of electrons being pumped on to it by the power source. Na + ions combine with the free. Positive lead(ii) ions are attracted to it. The electrode attached to the negative terminal of a battery is called a negative electrode, or cathode. Learn. Negative Electrode In The Electrolysis.
From www.slideshare.net
Gcse c5 electrolysis revison Negative Electrode In The Electrolysis The negative electrode during electrolysis is called the cathode. Find out the definitions and examples of anode and. Learn about electrolytic cells, which are devices that use electrical energy to drive nonspontaneous redox reactions. Positive lead(ii) ions are attracted to it. Cation is a positively charged ion which is attracted to the cathode. Learn how to predict the products of. Negative Electrode In The Electrolysis.
From www.worldatlas.com
What is the Difference Between a Cation and an Anion? WorldAtlas Negative Electrode In The Electrolysis Learn about electrolytic cells, which are devices that use electrical energy to drive nonspontaneous redox reactions. Find out the definitions and examples of anode and. Na + ions combine with the free. When they get there, 2. The negative electrode during electrolysis is called the cathode. Cathode is the negative electrode of an electrolysis cell. These negative electrons create a. Negative Electrode In The Electrolysis.
From en.ppt-online.org
Electrolysis online presentation Negative Electrode In The Electrolysis Learn how to predict the products of electrolysis of different. The cathode (the negative electrode) has an abundance of electrons being pumped on to it by the power source. Learn about electrolytic cells, which are devices that use electrical energy to drive nonspontaneous redox reactions. Learn about electrolysis, the decomposition of a compound using an electric current. Cathode is the. Negative Electrode In The Electrolysis.
From www.sciencenewsforstudents.org
Explainer What is an electrode? Science News for Students Negative Electrode In The Electrolysis The cathode (the negative electrode) has an abundance of electrons being pumped on to it by the power source. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. When they get there, 2. Cathode is the negative electrode of an electrolysis cell. Learn about electrolysis, the decomposition of. Negative Electrode In The Electrolysis.
From www.vedantu.com
Cathode and Anode Definition and Difference Between Anode and Cathode Negative Electrode In The Electrolysis Learn about electrolysis, the decomposition of a compound using an electric current. Positive lead(ii) ions are attracted to it. When they get there, 2. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. The negative electrode during electrolysis is called the cathode. Na + ions combine with the. Negative Electrode In The Electrolysis.
From userpartfrieda.z21.web.core.windows.net
Cathode Electrolyte Circuit Diagram Negative Electrode In The Electrolysis Electrolysis is a process in which an external circuit does work on a redox system to drive a nonspontaneous reaction. Cation is a positively charged ion which is attracted to the cathode. Positive lead(ii) ions are attracted to it. Cathode is the negative electrode of an electrolysis cell. The cathode (the negative electrode) has an abundance of electrons being pumped. Negative Electrode In The Electrolysis.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Negative Electrode In The Electrolysis Na + ions combine with the free. The negative electrode during electrolysis is called the cathode. Cation is a positively charged ion which is attracted to the cathode. When they get there, 2. Find out the definitions and examples of anode and. Learn about electrolysis, the decomposition of a compound using an electric current. Learn how to predict the products. Negative Electrode In The Electrolysis.
From www.dreamstime.com
Electrolysis Process Anode and Cathode Reactions Stock Illustration Negative Electrode In The Electrolysis Learn about electrolytic cells, which are devices that use electrical energy to drive nonspontaneous redox reactions. When they get there, 2. Learn how to predict the products of electrolysis of different. Positive lead(ii) ions are attracted to it. Learn about electrolysis, the decomposition of a compound using an electric current. Find out the definitions and examples of anode and. Cation. Negative Electrode In The Electrolysis.
From courses.lumenlearning.com
Electrolysis Chemistry Negative Electrode In The Electrolysis Cation is a positively charged ion which is attracted to the cathode. Learn about electrolysis, the decomposition of a compound using an electric current. Learn how to predict the products of electrolysis of different. Cathode is the negative electrode of an electrolysis cell. Find out the definitions and examples of anode and. The cathode (the negative electrode) has an abundance. Negative Electrode In The Electrolysis.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID6499367 Negative Electrode In The Electrolysis Cation is a positively charged ion which is attracted to the cathode. Positive lead(ii) ions are attracted to it. The negative electrode during electrolysis is called the cathode. Learn about electrolysis, the decomposition of a compound using an electric current. Electrolysis is a process in which an external circuit does work on a redox system to drive a nonspontaneous reaction.. Negative Electrode In The Electrolysis.
From www.elevise.co.uk
C4 J) Electrolysis Part 1 AQA Combined Science Trilogy Elevise Negative Electrode In The Electrolysis Learn about electrolytic cells, which are devices that use electrical energy to drive nonspontaneous redox reactions. Na + ions combine with the free. Learn about electrolysis, the decomposition of a compound using an electric current. Learn how to predict the products of electrolysis of different. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive. Negative Electrode In The Electrolysis.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Negative Electrode In The Electrolysis Positive lead(ii) ions are attracted to it. Find out the definitions and examples of anode and. Learn about electrolytic cells, which are devices that use electrical energy to drive nonspontaneous redox reactions. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. Cation is a positively charged ion which. Negative Electrode In The Electrolysis.