What Is Equivalence Point Of A Titration . In a titration, it is. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Sorting out some confusing terms. Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. The equivalence point of a titration.
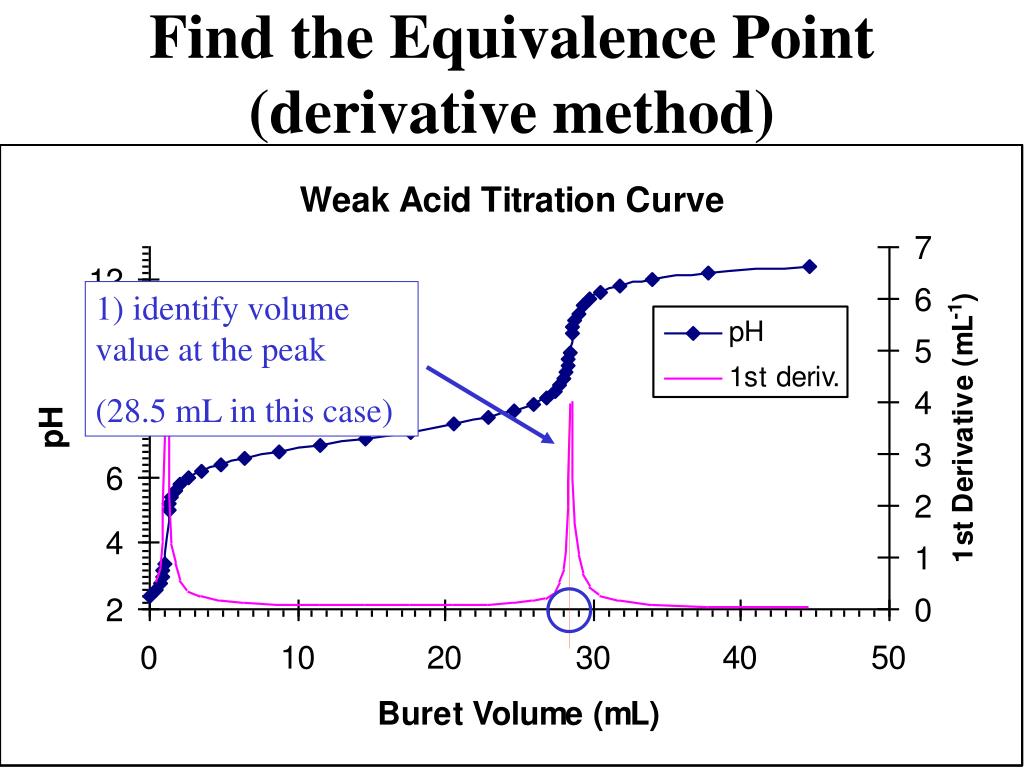
from www.slideserve.com
A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. Sorting out some confusing terms. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. In a titration, it is. The equivalence point of a titration. Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically.
PPT How to Interpret Titration Curves PowerPoint Presentation, free
What Is Equivalence Point Of A Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The equivalence point of a titration. Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Sorting out some confusing terms. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. In a titration, it is. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps What Is Equivalence Point Of A Titration The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. Sorting out some confusing terms. In a titration, it is. Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The equivalence point of a. What Is Equivalence Point Of A Titration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry What Is Equivalence Point Of A Titration When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. The equivalence point of a titration. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. Sorting out some confusing. What Is Equivalence Point Of A Titration.
From schoolbag.info
If the acetic acid being titrated here were replaced by hydrochloric What Is Equivalence Point Of A Titration Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The equivalence point of a titration. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. When 25 ml of titrant has been added (the equivalence point),. What Is Equivalence Point Of A Titration.
From www.bartleby.com
Answered Ca(OH)2 Titration Data for Ksp 12… bartleby What Is Equivalence Point Of A Titration The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. Sorting out some confusing terms. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. A point of equivalence in a titration refers to a point. What Is Equivalence Point Of A Titration.
From www.writework.com
Titration of amino acids WriteWork What Is Equivalence Point Of A Titration Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the. What Is Equivalence Point Of A Titration.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe What Is Equivalence Point Of A Titration The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. In a titration, it is. The equivalence point of a. What Is Equivalence Point Of A Titration.
From www.youtube.com
Titration Curves, Equivalence Point YouTube What Is Equivalence Point Of A Titration Sorting out some confusing terms. The equivalence point of a titration. In a titration, it is. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution.. What Is Equivalence Point Of A Titration.
From ar.inspiredpencil.com
H3po4 Titration Curve What Is Equivalence Point Of A Titration The equivalence point of a titration. Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. A point of equivalence in a titration refers to. What Is Equivalence Point Of A Titration.
From www.youtube.com
Strong acid / strong base titration pH at equivalence point YouTube What Is Equivalence Point Of A Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Sorting out some confusing terms. The equivalence point of a titration. The equivalence point or stoichiometric point is. What Is Equivalence Point Of A Titration.
From www.youtube.com
Weak acid / strong base titration pH at equivalence point YouTube What Is Equivalence Point Of A Titration The equivalence point of a titration. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. Sorting out some confusing terms. When. What Is Equivalence Point Of A Titration.
From www.numerade.com
SOLVEDThe graph shows the titration curves for two What Is Equivalence Point Of A Titration The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In a titration, it is. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. When 25 ml of titrant has been added (the equivalence point),. What Is Equivalence Point Of A Titration.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts What Is Equivalence Point Of A Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. When 25 ml of titrant has been added (the equivalence point), the. What Is Equivalence Point Of A Titration.
From mungfali.com
Half Equivalence Point On Titration Curve What Is Equivalence Point Of A Titration The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The equivalence point of a chemical reaction is the point at which. What Is Equivalence Point Of A Titration.
From www.youtube.com
Titration curves in details. Equivalence point. Half equivalence point What Is Equivalence Point Of A Titration The equivalence point of a titration. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. Sorting out some confusing terms. The. What Is Equivalence Point Of A Titration.
From www.chemistrystudent.com
Finding Ka using a Titration Curve (A2level) ChemistryStudent What Is Equivalence Point Of A Titration In a titration, it is. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. The equivalence point of a titration. A point of equivalence in. What Is Equivalence Point Of A Titration.
From www.numerade.com
SOLVEDWhat is a titration? What is the equivalence point? What Is Equivalence Point Of A Titration The equivalence point of a titration. In a titration, it is. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Titration calculations at the equivalence point in. What Is Equivalence Point Of A Titration.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept What Is Equivalence Point Of A Titration In a titration, it is. Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. The equivalence point of a titration. The equivalence point or. What Is Equivalence Point Of A Titration.
From www.youtube.com
Titrations and the M1V1=M2V2 Math at The Equivalence Point YouTube What Is Equivalence Point Of A Titration Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are. What Is Equivalence Point Of A Titration.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii What Is Equivalence Point Of A Titration The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. A point of equivalence in a titration refers to a point at which the added titrant. What Is Equivalence Point Of A Titration.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 What Is Equivalence Point Of A Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. In a titration, it is. Sorting out some confusing terms. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. The equivalence. What Is Equivalence Point Of A Titration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry What Is Equivalence Point Of A Titration The equivalence point of a titration. Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Sorting out some confusing terms. In a titration, it is. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the. What Is Equivalence Point Of A Titration.
From www.youtube.com
Finding equivalence point for Monoprotic acid YouTube What Is Equivalence Point Of A Titration Sorting out some confusing terms. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. In a titration, it is. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. Titration calculations. What Is Equivalence Point Of A Titration.
From mavink.com
What Is The Equivalence Point Of A Titration What Is Equivalence Point Of A Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. Sorting out some confusing terms. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. The equivalence point of a chemical reaction. What Is Equivalence Point Of A Titration.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co What Is Equivalence Point Of A Titration The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Sorting out some confusing terms. The equivalence point of a chemical reaction is the point. What Is Equivalence Point Of A Titration.
From www.youtube.com
Titration Weak base/Strong acid Equivalence Point YouTube What Is Equivalence Point Of A Titration The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. Sorting out some confusing terms. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. A point of equivalence in a titration refers to a point. What Is Equivalence Point Of A Titration.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube What Is Equivalence Point Of A Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The equivalence point of a titration. In a titration, it is. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. The. What Is Equivalence Point Of A Titration.
From schoolbag.info
Figure 10.9. Monoprotic Strong Acid and Strong Base Titration Curve A What Is Equivalence Point Of A Titration Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In a titration, it is. The equivalence point of a titration. When 25 ml of titrant has been added (the equivalence. What Is Equivalence Point Of A Titration.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators What Is Equivalence Point Of A Titration The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. The equivalence point of a titration. The equivalence point or stoichiometric point is the point in. What Is Equivalence Point Of A Titration.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts What Is Equivalence Point Of A Titration Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The equivalence point of a titration. When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. Sorting out some confusing terms. The equivalence point or. What Is Equivalence Point Of A Titration.
From www.chegg.com
Solved What is the approximate pH at the equivalence point What Is Equivalence Point Of A Titration In a titration, it is. The equivalence point of a titration. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. A point of equivalence in. What Is Equivalence Point Of A Titration.
From saylordotorg.github.io
AcidBase Titrations What Is Equivalence Point Of A Titration The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. Sorting out some confusing terms. Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base. A point of equivalence in a titration refers to a. What Is Equivalence Point Of A Titration.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps What Is Equivalence Point Of A Titration The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In a titration, it is. Sorting out some confusing terms. Titration calculations at the equivalence point. What Is Equivalence Point Of A Titration.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent What Is Equivalence Point Of A Titration When 25 ml of titrant has been added (the equivalence point), the ph is well above the upper limit and the solution will appear yellow. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. The equivalence point of a titration. The equivalence point of. What Is Equivalence Point Of A Titration.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free What Is Equivalence Point Of A Titration The equivalence point of a titration. In a titration, it is. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. Sorting out some confusing terms. When 25. What Is Equivalence Point Of A Titration.
From www.chegg.com
Solved Identify the equivalence point on the titration curve What Is Equivalence Point Of A Titration Sorting out some confusing terms. Titration calculations at the equivalence point in a neutralization, the moles of acid are equal to the moles of base. In a titration, it is. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. When 25 ml of titrant. What Is Equivalence Point Of A Titration.