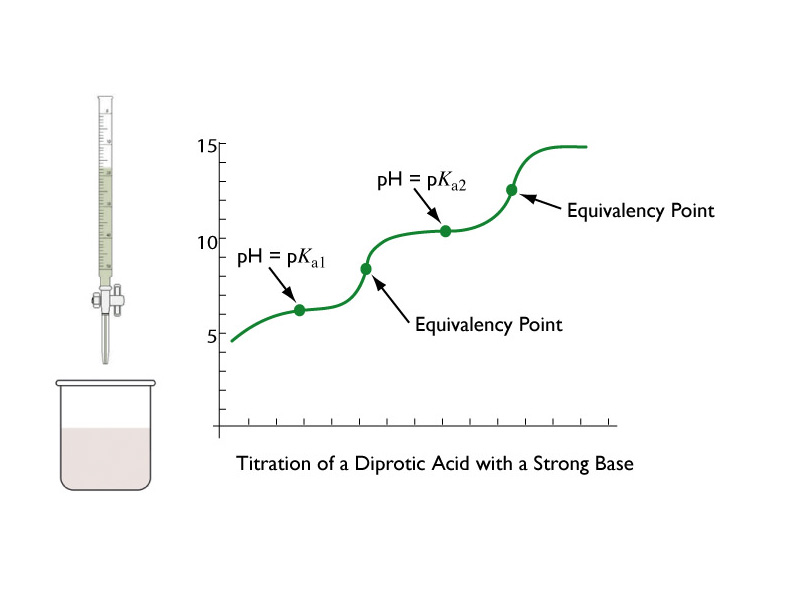
Titration Graph Diprotic . with the titration of a diprotic acid, we now have to keep in mind the existence of 2 equivalence points as well. A diprotic acid is titrated with naoh solution of known concentration. if the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. Molecular weight (or molar mass) is found in g in this lab you need to titrate 25 ml of the analyte and make ph measurements every 1 ml except when you are near each of the two equivalence points, where you need to take make a measurement every 0.1 ml for the range from one ml below to one ml above the equivalence point. the equivalence point on the graph is where all of the starting solution (usually an acid) has been neutralized by the titrant. All acid titration curves follow the same basic shapes. In the beginning, the solution has a the primary purpose of this experiment is to identify an unknown diprotic acid by finding its molecular weight.
from mavink.com
in this lab you need to titrate 25 ml of the analyte and make ph measurements every 1 ml except when you are near each of the two equivalence points, where you need to take make a measurement every 0.1 ml for the range from one ml below to one ml above the equivalence point. All acid titration curves follow the same basic shapes. the equivalence point on the graph is where all of the starting solution (usually an acid) has been neutralized by the titrant. with the titration of a diprotic acid, we now have to keep in mind the existence of 2 equivalence points as well. if the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. In the beginning, the solution has a the primary purpose of this experiment is to identify an unknown diprotic acid by finding its molecular weight. A diprotic acid is titrated with naoh solution of known concentration. Molecular weight (or molar mass) is found in g
Diprotic Acid Titration Curve
Titration Graph Diprotic In the beginning, the solution has a Molecular weight (or molar mass) is found in g the equivalence point on the graph is where all of the starting solution (usually an acid) has been neutralized by the titrant. All acid titration curves follow the same basic shapes. In the beginning, the solution has a A diprotic acid is titrated with naoh solution of known concentration. in this lab you need to titrate 25 ml of the analyte and make ph measurements every 1 ml except when you are near each of the two equivalence points, where you need to take make a measurement every 0.1 ml for the range from one ml below to one ml above the equivalence point. the primary purpose of this experiment is to identify an unknown diprotic acid by finding its molecular weight. with the titration of a diprotic acid, we now have to keep in mind the existence of 2 equivalence points as well. if the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve.
From ar.inspiredpencil.com
Diagram Of Acid Base Titration Titration Graph Diprotic in this lab you need to titrate 25 ml of the analyte and make ph measurements every 1 ml except when you are near each of the two equivalence points, where you need to take make a measurement every 0.1 ml for the range from one ml below to one ml above the equivalence point. with the titration. Titration Graph Diprotic.
From www.chegg.com
Solved Consider the titration curve for a diprotic acid 12 Titration Graph Diprotic in this lab you need to titrate 25 ml of the analyte and make ph measurements every 1 ml except when you are near each of the two equivalence points, where you need to take make a measurement every 0.1 ml for the range from one ml below to one ml above the equivalence point. Molecular weight (or molar. Titration Graph Diprotic.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Graph Diprotic Molecular weight (or molar mass) is found in g In the beginning, the solution has a in this lab you need to titrate 25 ml of the analyte and make ph measurements every 1 ml except when you are near each of the two equivalence points, where you need to take make a measurement every 0.1 ml for the. Titration Graph Diprotic.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Graph Diprotic the primary purpose of this experiment is to identify an unknown diprotic acid by finding its molecular weight. with the titration of a diprotic acid, we now have to keep in mind the existence of 2 equivalence points as well. A diprotic acid is titrated with naoh solution of known concentration. in this lab you need to. Titration Graph Diprotic.
From www.numerade.com
SOLVEDPrelab Diprotic Acid Titration The graph bclou shows the Titration Graph Diprotic in this lab you need to titrate 25 ml of the analyte and make ph measurements every 1 ml except when you are near each of the two equivalence points, where you need to take make a measurement every 0.1 ml for the range from one ml below to one ml above the equivalence point. the equivalence point. Titration Graph Diprotic.
From exyuokede.blob.core.windows.net
Titration Graph Buffer at Mark Paras blog Titration Graph Diprotic Molecular weight (or molar mass) is found in g In the beginning, the solution has a in this lab you need to titrate 25 ml of the analyte and make ph measurements every 1 ml except when you are near each of the two equivalence points, where you need to take make a measurement every 0.1 ml for the. Titration Graph Diprotic.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Graph Diprotic All acid titration curves follow the same basic shapes. A diprotic acid is titrated with naoh solution of known concentration. the equivalence point on the graph is where all of the starting solution (usually an acid) has been neutralized by the titrant. with the titration of a diprotic acid, we now have to keep in mind the existence. Titration Graph Diprotic.
From www.chegg.com
Solved Prelab Diprotic Acid Titration 1. The graph below Titration Graph Diprotic the equivalence point on the graph is where all of the starting solution (usually an acid) has been neutralized by the titrant. A diprotic acid is titrated with naoh solution of known concentration. Molecular weight (or molar mass) is found in g with the titration of a diprotic acid, we now have to keep in mind the existence. Titration Graph Diprotic.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Graph Diprotic Molecular weight (or molar mass) is found in g A diprotic acid is titrated with naoh solution of known concentration. if the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. the equivalence point on the graph is where all of. Titration Graph Diprotic.
From mavink.com
Diprotic Acid Titration Curve Titration Graph Diprotic in this lab you need to titrate 25 ml of the analyte and make ph measurements every 1 ml except when you are near each of the two equivalence points, where you need to take make a measurement every 0.1 ml for the range from one ml below to one ml above the equivalence point. Molecular weight (or molar. Titration Graph Diprotic.
From www.chegg.com
Solved The graph shown is a titration of a diprotic amino Titration Graph Diprotic in this lab you need to titrate 25 ml of the analyte and make ph measurements every 1 ml except when you are near each of the two equivalence points, where you need to take make a measurement every 0.1 ml for the range from one ml below to one ml above the equivalence point. Molecular weight (or molar. Titration Graph Diprotic.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Titration Graph Diprotic if the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. A diprotic acid is titrated with naoh solution of known concentration. In the beginning, the solution has a Molecular weight (or molar mass) is found in g the primary purpose. Titration Graph Diprotic.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Graph Diprotic in this lab you need to titrate 25 ml of the analyte and make ph measurements every 1 ml except when you are near each of the two equivalence points, where you need to take make a measurement every 0.1 ml for the range from one ml below to one ml above the equivalence point. the primary purpose. Titration Graph Diprotic.
From webmis.highland.cc.il.us
AcidBase Titrations Titration Graph Diprotic in this lab you need to titrate 25 ml of the analyte and make ph measurements every 1 ml except when you are near each of the two equivalence points, where you need to take make a measurement every 0.1 ml for the range from one ml below to one ml above the equivalence point. with the titration. Titration Graph Diprotic.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Graph Diprotic A diprotic acid is titrated with naoh solution of known concentration. in this lab you need to titrate 25 ml of the analyte and make ph measurements every 1 ml except when you are near each of the two equivalence points, where you need to take make a measurement every 0.1 ml for the range from one ml below. Titration Graph Diprotic.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Graph Diprotic the equivalence point on the graph is where all of the starting solution (usually an acid) has been neutralized by the titrant. if the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. the primary purpose of this experiment is. Titration Graph Diprotic.
From www.researchgate.net
Titration behavior of a diprotic acid with an interaction energy W Titration Graph Diprotic the equivalence point on the graph is where all of the starting solution (usually an acid) has been neutralized by the titrant. the primary purpose of this experiment is to identify an unknown diprotic acid by finding its molecular weight. if the ph of an acid solution is plotted against the amount of base added during a. Titration Graph Diprotic.
From mavink.com
Diprotic Acid Titration Curve Titration Graph Diprotic Molecular weight (or molar mass) is found in g with the titration of a diprotic acid, we now have to keep in mind the existence of 2 equivalence points as well. A diprotic acid is titrated with naoh solution of known concentration. in this lab you need to titrate 25 ml of the analyte and make ph measurements. Titration Graph Diprotic.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Graph Diprotic All acid titration curves follow the same basic shapes. Molecular weight (or molar mass) is found in g A diprotic acid is titrated with naoh solution of known concentration. the equivalence point on the graph is where all of the starting solution (usually an acid) has been neutralized by the titrant. with the titration of a diprotic acid,. Titration Graph Diprotic.
From chemistryguru.com.sg
Titration Curve of Amino Acid Titration Graph Diprotic A diprotic acid is titrated with naoh solution of known concentration. the primary purpose of this experiment is to identify an unknown diprotic acid by finding its molecular weight. if the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. In. Titration Graph Diprotic.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Graph Diprotic All acid titration curves follow the same basic shapes. the equivalence point on the graph is where all of the starting solution (usually an acid) has been neutralized by the titrant. Molecular weight (or molar mass) is found in g with the titration of a diprotic acid, we now have to keep in mind the existence of 2. Titration Graph Diprotic.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Graph Diprotic In the beginning, the solution has a the primary purpose of this experiment is to identify an unknown diprotic acid by finding its molecular weight. with the titration of a diprotic acid, we now have to keep in mind the existence of 2 equivalence points as well. in this lab you need to titrate 25 ml of. Titration Graph Diprotic.
From forums.studentdoctor.net
Confusion about titration of polyprotic acids Student Doctor Network Titration Graph Diprotic in this lab you need to titrate 25 ml of the analyte and make ph measurements every 1 ml except when you are near each of the two equivalence points, where you need to take make a measurement every 0.1 ml for the range from one ml below to one ml above the equivalence point. the equivalence point. Titration Graph Diprotic.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Graph Diprotic the equivalence point on the graph is where all of the starting solution (usually an acid) has been neutralized by the titrant. the primary purpose of this experiment is to identify an unknown diprotic acid by finding its molecular weight. if the ph of an acid solution is plotted against the amount of base added during a. Titration Graph Diprotic.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Graph Diprotic A diprotic acid is titrated with naoh solution of known concentration. if the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. In the beginning, the solution has a the primary purpose of this experiment is to identify an unknown diprotic. Titration Graph Diprotic.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Graph Diprotic the equivalence point on the graph is where all of the starting solution (usually an acid) has been neutralized by the titrant. with the titration of a diprotic acid, we now have to keep in mind the existence of 2 equivalence points as well. A diprotic acid is titrated with naoh solution of known concentration. in this. Titration Graph Diprotic.
From godwinaces1963.blogspot.com
Weak Diprotic Acid Titration Curve Godwin Aces1963 Titration Graph Diprotic Molecular weight (or molar mass) is found in g with the titration of a diprotic acid, we now have to keep in mind the existence of 2 equivalence points as well. the primary purpose of this experiment is to identify an unknown diprotic acid by finding its molecular weight. the equivalence point on the graph is where. Titration Graph Diprotic.
From www.numerade.com
SOLVED Prelab Diprotic Acid Titration NOH graph / below shows the Titration Graph Diprotic with the titration of a diprotic acid, we now have to keep in mind the existence of 2 equivalence points as well. in this lab you need to titrate 25 ml of the analyte and make ph measurements every 1 ml except when you are near each of the two equivalence points, where you need to take make. Titration Graph Diprotic.
From boisestate.pressbooks.pub
14.7 AcidBase Titrations General Chemistry 1 & 2 Titration Graph Diprotic in this lab you need to titrate 25 ml of the analyte and make ph measurements every 1 ml except when you are near each of the two equivalence points, where you need to take make a measurement every 0.1 ml for the range from one ml below to one ml above the equivalence point. if the ph. Titration Graph Diprotic.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Graph Diprotic the equivalence point on the graph is where all of the starting solution (usually an acid) has been neutralized by the titrant. Molecular weight (or molar mass) is found in g All acid titration curves follow the same basic shapes. in this lab you need to titrate 25 ml of the analyte and make ph measurements every 1. Titration Graph Diprotic.
From godwinaces1963.blogspot.com
Weak Diprotic Acid Titration Curve Godwin Aces1963 Titration Graph Diprotic in this lab you need to titrate 25 ml of the analyte and make ph measurements every 1 ml except when you are near each of the two equivalence points, where you need to take make a measurement every 0.1 ml for the range from one ml below to one ml above the equivalence point. with the titration. Titration Graph Diprotic.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Graph Diprotic the equivalence point on the graph is where all of the starting solution (usually an acid) has been neutralized by the titrant. with the titration of a diprotic acid, we now have to keep in mind the existence of 2 equivalence points as well. All acid titration curves follow the same basic shapes. if the ph of. Titration Graph Diprotic.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Graph Diprotic in this lab you need to titrate 25 ml of the analyte and make ph measurements every 1 ml except when you are near each of the two equivalence points, where you need to take make a measurement every 0.1 ml for the range from one ml below to one ml above the equivalence point. Molecular weight (or molar. Titration Graph Diprotic.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Graph Diprotic if the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. the primary purpose of this experiment is to identify an unknown diprotic acid by finding its molecular weight. In the beginning, the solution has a Molecular weight (or molar mass). Titration Graph Diprotic.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Graph Diprotic Molecular weight (or molar mass) is found in g if the ph of an acid solution is plotted against the amount of base added during a titration, the shape of the graph is called a titration curve. In the beginning, the solution has a with the titration of a diprotic acid, we now have to keep in mind. Titration Graph Diprotic.