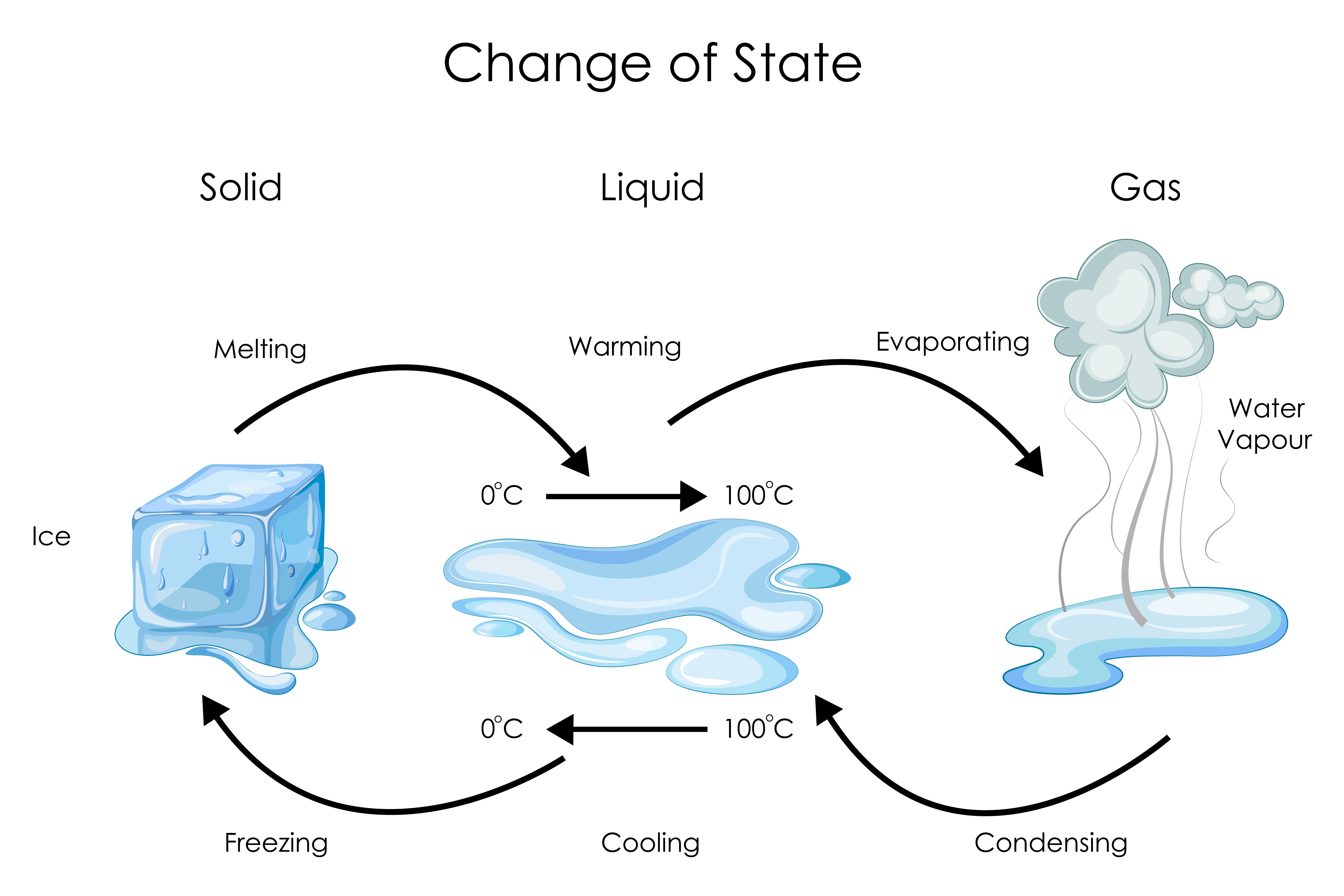
Solid To Liquid Is Called Change . The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. This change is called a phase transition or phase change. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. Every element and substance can transition from one phase to another at a. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The process by which a substance. Changes in temperature and pressure causes matter to change from one state to another. The process by which a substance changes from the liquid phase to the solid phase is known as freezing.
from primaryleap.co.uk
Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. Changes in temperature and pressure causes matter to change from one state to another. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. The process by which a substance changes from the liquid phase to the solid phase is known as freezing. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. This change is called a phase transition or phase change. The process by which a substance. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Every element and substance can transition from one phase to another at a.
Chemistry States Of Matter Level 2 activity for kids PrimaryLeap.co.uk
Solid To Liquid Is Called Change The process by which a substance changes from the liquid phase to the solid phase is known as freezing. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Changes in temperature and pressure causes matter to change from one state to another. Every element and substance can transition from one phase to another at a. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. This change is called a phase transition or phase change. The process by which a substance. The process by which a substance changes from the liquid phase to the solid phase is known as freezing. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and.
From fyoecbagh.blob.core.windows.net
Conversion Of Liquid Into Solid Is Called at Roger Feliciano blog Solid To Liquid Is Called Change This change is called a phase transition or phase change. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. The process by which a substance changes from the liquid phase to the solid phase is known as freezing. Every element and substance can transition from one phase to another. Solid To Liquid Is Called Change.
From www.gkseries.com
When a solid, liquid, or a gas changes from one physical state to another, the change is called Solid To Liquid Is Called Change Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The process by which a substance changes from the liquid phase to the solid phase is known as freezing. The process by which a substance. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation.. Solid To Liquid Is Called Change.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change Solid To Liquid Is Called Change The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. The process by which a substance. Changes in temperature and pressure causes matter to change from one state to another. The process by which a substance changes from the liquid phase to the solid phase is known as freezing. The direct conversion of a. Solid To Liquid Is Called Change.
From tutormate.in
Matter In Our Surrounding Tutormate Solid To Liquid Is Called Change This change is called a phase transition or phase change. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. Changes in temperature and pressure causes matter to change from one state to another. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and.. Solid To Liquid Is Called Change.
From courses.lumenlearning.com
Solid to Gas Phase Transition Introduction to Chemistry Solid To Liquid Is Called Change The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. The process by which a substance changes from the liquid phase to the solid phase is known as freezing. The process by which a substance. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid. Solid To Liquid Is Called Change.
From primaryleap.co.uk
Chemistry States Of Matter Level 2 activity for kids PrimaryLeap.co.uk Solid To Liquid Is Called Change Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Changes in temperature and pressure causes matter to change from one state to another. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. The process by which a substance changes from the liquid phase to. Solid To Liquid Is Called Change.
From quizlet.com
States and Changes of Matter Diagram Quizlet Solid To Liquid Is Called Change The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. This change is called a phase transition or phase change. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. The process by which a substance. The process by which a substance changes from the liquid phase. Solid To Liquid Is Called Change.
From www.bartleby.com
Answered The changing of a liquid to a solid is… bartleby Solid To Liquid Is Called Change Every element and substance can transition from one phase to another at a. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. This change is called a phase transition or phase change. Changes in temperature and pressure causes matter to change from one state to another. Under some circumstances, the solid. Solid To Liquid Is Called Change.
From www.numerade.com
Fill in the chart below to identify the name of the process that explains the state of change Solid To Liquid Is Called Change Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. This change is called a phase transition or phase change. The process by which a substance. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. The process by which a substance. Solid To Liquid Is Called Change.
From www.pinterest.com
States of Matter (solids, liquids and gases) The Chemistry Journey T... States of matter Solid To Liquid Is Called Change Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. The process by which a substance changes from the liquid phase to the solid phase is known as freezing. Changes in temperature and pressure causes matter to change from one state to another. Every element and substance can transition from. Solid To Liquid Is Called Change.
From www.ase.org.uk
Solids, liquids and gases Solid To Liquid Is Called Change Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. Under some circumstances, the solid phase can transition directly. Solid To Liquid Is Called Change.
From www.dreamstime.com
Illustration for Changes of State between Solid, Liquid and Gas Stock Illustration Solid To Liquid Is Called Change Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. The process by which a substance. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. Every element and. Solid To Liquid Is Called Change.
From allaboutchemistry123.blogspot.com
What are solid, liquid, and gases? All About Chemistry Solid To Liquid Is Called Change The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Changes in temperature and pressure causes matter to change from one state to another. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. The amount of energy required to sublime 1 mol. Solid To Liquid Is Called Change.
From www.britannica.com
phase Definition & Facts Britannica Solid To Liquid Is Called Change This change is called a phase transition or phase change. Every element and substance can transition from one phase to another at a. Changes in temperature and pressure causes matter to change from one state to another. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The process by which a. Solid To Liquid Is Called Change.
From shaunmwilliams.com
Chapter 1 Presentation Solid To Liquid Is Called Change The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Changes in temperature and pressure causes matter to change from one state to another. Every element and substance can transition from one phase to another at a. The process by which a substance changes from the liquid phase to the solid phase is. Solid To Liquid Is Called Change.
From slideplayer.com
Phases of Matter Phase Changes Heating and Cooling Curves ppt download Solid To Liquid Is Called Change The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. This change is called a phase transition or phase change. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Changes in temperature and pressure causes matter to change from one state to another. The process. Solid To Liquid Is Called Change.
From www.exploringnature.org
Phases of Matter Gas, Liquids, Solids Solid To Liquid Is Called Change The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. This change is called a phase transition or phase change. Changes in temperature and pressure causes matter to change from one state to another. The. Solid To Liquid Is Called Change.
From itinerantmission.blogspot.com
Itinerant Mission 3 Physical States of Matter Solid Liquid Gas Solid To Liquid Is Called Change The process by which a substance changes from the liquid phase to the solid phase is known as freezing. Changes in temperature and pressure causes matter to change from one state to another. The process by which a substance. Every element and substance can transition from one phase to another at a. The direct conversion of a solid to a. Solid To Liquid Is Called Change.
From sciencenotes.org
States of Matter Solid To Liquid Is Called Change Changes in temperature and pressure causes matter to change from one state to another. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. Phase transition is when a substance changes from a solid,. Solid To Liquid Is Called Change.
From www.gkseries.com
The process of changing the solid into a liquid by supplying the heat is called as Solid To Liquid Is Called Change Changes in temperature and pressure causes matter to change from one state to another. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. Every element and substance can transition from one phase to another at. Solid To Liquid Is Called Change.
From www.snexplores.org
Explainer What are the different states of matter? Solid To Liquid Is Called Change The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. The process by which a substance changes from the liquid phase to the solid phase is known as freezing. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. The direct conversion of a. Solid To Liquid Is Called Change.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change Solid To Liquid Is Called Change This change is called a phase transition or phase change. The process by which a substance. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. The process by which a substance changes from the liquid phase to the solid phase is known as freezing. Every element and substance can transition from one. Solid To Liquid Is Called Change.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Solid To Liquid Is Called Change The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. The process by which a substance. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. Phase transition is when a substance changes from a solid, liquid, or gas state to a different. Solid To Liquid Is Called Change.
From kids.britannica.com
solid Students Britannica Kids Homework Help Solid To Liquid Is Called Change Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The process by which a substance. The process by which a substance changes from the liquid phase to the solid phase is known as freezing. Under some circumstances, the solid phase can transition directly to the gas phase without going through a. Solid To Liquid Is Called Change.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Solid To Liquid Is Called Change The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Every element and substance can transition from one phase to another at a. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. This change is called a phase transition or phase change. The process. Solid To Liquid Is Called Change.
From www.chegg.com
Solved 28. The process of changing from a liquid to a solid Solid To Liquid Is Called Change Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Changes in temperature and pressure causes matter to change from one state to another. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. This change is called a phase transition or phase change. The process. Solid To Liquid Is Called Change.
From www.vecteezy.com
Three different States of matter solid, liquid and gasuas state. Inter change of state of matter Solid To Liquid Is Called Change Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Every element and substance can transition from one phase to another at a. This change is called a phase transition or phase change.. Solid To Liquid Is Called Change.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning Solid To Liquid Is Called Change Changes in temperature and pressure causes matter to change from one state to another. Every element and substance can transition from one phase to another at a. The process by which a substance changes from the liquid phase to the solid phase is known as freezing. Under some circumstances, the solid phase can transition directly to the gas phase without. Solid To Liquid Is Called Change.
From www.thoughtco.com
List of Phase Changes Between States of Matter Solid To Liquid Is Called Change The process by which a substance. This change is called a phase transition or phase change. The process by which a substance changes from the liquid phase to the solid phase is known as freezing. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. The amount of energy required to sublime 1. Solid To Liquid Is Called Change.
From sciencenotes.org
Phase Changes of Matter (Phase Transitions) Solid To Liquid Is Called Change The process by which a substance. The process by which a substance changes from the liquid phase to the solid phase is known as freezing. This change is called a phase transition or phase change. Changes in temperature and pressure causes matter to change from one state to another. Under some circumstances, the solid phase can transition directly to the. Solid To Liquid Is Called Change.
From byjus.com
The process through which a solid directly changes to a gaseous state without passing through a Solid To Liquid Is Called Change The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Every element and substance can transition from one phase to another at a. The amount of energy required to sublime 1 mol of a pure solid is the enthalpy. The process by which a substance changes from the liquid phase to the solid. Solid To Liquid Is Called Change.
From mungfali.com
Solid Liquid Gas Anchor Chart Solid To Liquid Is Called Change The process by which a substance. Changes in temperature and pressure causes matter to change from one state to another. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. The process by. Solid To Liquid Is Called Change.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change Solid To Liquid Is Called Change Changes in temperature and pressure causes matter to change from one state to another. The process by which a substance changes from the liquid phase to the solid phase is known as freezing. This change is called a phase transition or phase change. Under some circumstances, the solid phase can transition directly to the gas phase without going through a. Solid To Liquid Is Called Change.
From owlcation.com
What Is the Particle Model? A Guide to Solids, Liquids and Gases Owlcation Solid To Liquid Is Called Change Every element and substance can transition from one phase to another at a. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. The direct conversion of a solid to a gas, without an intervening liquid phase, is called sublimation. Phase transition is when a substance changes from a solid,. Solid To Liquid Is Called Change.
From slideplayer.com
Unit 1 Lesson 6 Changes of State ppt download Solid To Liquid Is Called Change Changes in temperature and pressure causes matter to change from one state to another. Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and. The process by which a substance changes from the liquid phase to the solid phase is known as freezing. Phase transition is when a substance changes. Solid To Liquid Is Called Change.