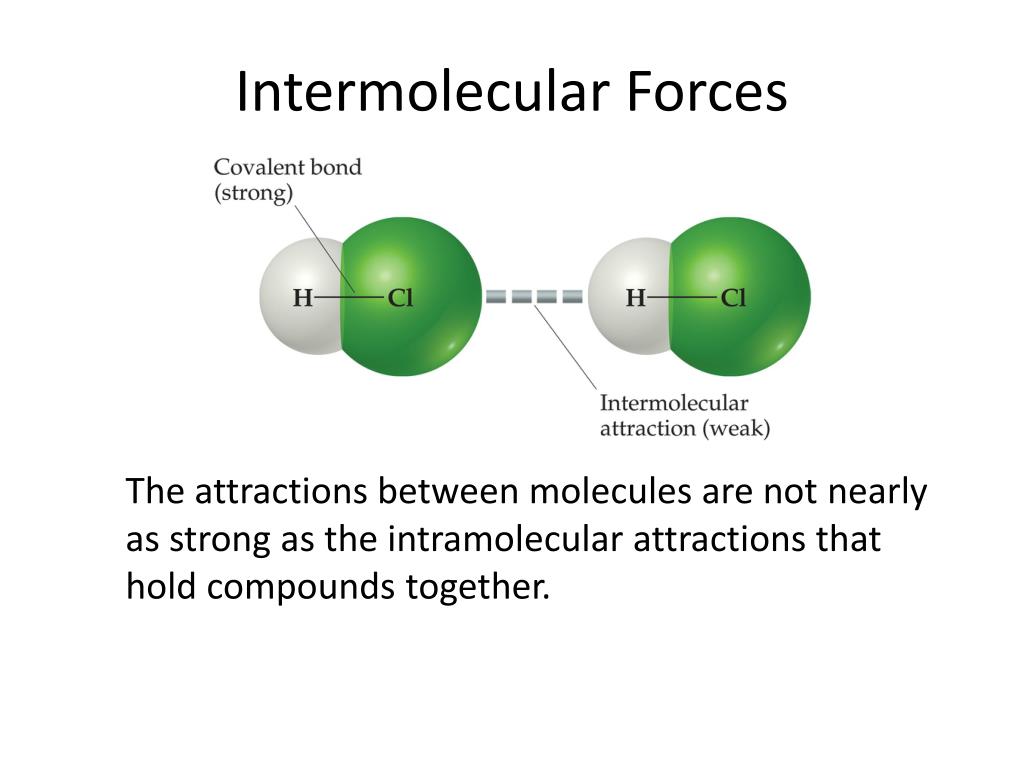
Intermolecular Forces In Bromine . Using a flowchart to guide. Compare the relative strengths of intermolecular forces; Identify the types of intermolecular forces experienced by specific molecules based on their structures; All of the attractive forces between. Identify the different types of intermolecular forces. Let’s look at them individually, from strongest to weakest. The extent to which one substance will dissolve in another is determined by several factors, including the types and relative strengths of. Like covalent and ionic bonds, intermolecular. Distinguish between the following three types of intermolecular forces: Intermolecular forces are electrostatic in nature; In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/ molecular bromine). Relate the physical properties of a substance to the strength of attractive forces. That is, they arise from the interaction between positively and negatively charged species. Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. The four key intermolecular forces are as follows:
from www.slideserve.com
In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/ molecular bromine). Relate the physical properties of a substance to the strength of attractive forces. That is, they arise from the interaction between positively and negatively charged species. Identify the different types of intermolecular forces. Compare the relative strengths of intermolecular forces; Distinguish between the following three types of intermolecular forces: Intermolecular forces are electrostatic in nature; Identify the types of intermolecular forces experienced by specific molecules based on their structures; All of the attractive forces between. Let’s look at them individually, from strongest to weakest.
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint Presentation ID2275843
Intermolecular Forces In Bromine The extent to which one substance will dissolve in another is determined by several factors, including the types and relative strengths of. Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. All of the attractive forces between. Like covalent and ionic bonds, intermolecular. Intermolecular forces are electrostatic in nature; The four key intermolecular forces are as follows: Relate the physical properties of a substance to the strength of attractive forces. That is, they arise from the interaction between positively and negatively charged species. In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/ molecular bromine). Using a flowchart to guide. The extent to which one substance will dissolve in another is determined by several factors, including the types and relative strengths of. Distinguish between the following three types of intermolecular forces: Let’s look at them individually, from strongest to weakest. Identify the types of intermolecular forces experienced by specific molecules based on their structures; Identify the different types of intermolecular forces. Compare the relative strengths of intermolecular forces;
From slideplayer.com
Intermolecular Forces ppt download Intermolecular Forces In Bromine Distinguish between the following three types of intermolecular forces: Identify the types of intermolecular forces experienced by specific molecules based on their structures; Relate the physical properties of a substance to the strength of attractive forces. Compare the relative strengths of intermolecular forces; In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/ molecular bromine). The four key. Intermolecular Forces In Bromine.
From www.numerade.com
SOLVED List the intermolecular forces present in hydrogen halides. Explain why hydrogen Intermolecular Forces In Bromine Using a flowchart to guide. Let’s look at them individually, from strongest to weakest. Relate the physical properties of a substance to the strength of attractive forces. Like covalent and ionic bonds, intermolecular. Distinguish between the following three types of intermolecular forces: Intermolecular forces are electrostatic in nature; In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/. Intermolecular Forces In Bromine.
From www.researchgate.net
(a) Intermolecular bromine/p interaction in the complex; (b) formation... Download Scientific Intermolecular Forces In Bromine That is, they arise from the interaction between positively and negatively charged species. The four key intermolecular forces are as follows: Distinguish between the following three types of intermolecular forces: Using a flowchart to guide. Compare the relative strengths of intermolecular forces; All of the attractive forces between. Identify the different types of intermolecular forces. The extent to which one. Intermolecular Forces In Bromine.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint Presentation ID2275843 Intermolecular Forces In Bromine Compare the relative strengths of intermolecular forces; Intermolecular forces are electrostatic in nature; Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. Like covalent and ionic bonds, intermolecular. Let’s look at them individually, from strongest to weakest. Identify the different types of intermolecular forces. The extent to which one substance will dissolve in another is determined. Intermolecular Forces In Bromine.
From www.youtube.com
Intermolecular Forces for Br2 (Diatomic Bromine) YouTube Intermolecular Forces In Bromine The extent to which one substance will dissolve in another is determined by several factors, including the types and relative strengths of. Compare the relative strengths of intermolecular forces; All of the attractive forces between. Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/. Intermolecular Forces In Bromine.
From www.coursehero.com
[Solved] Intermolecular Forces & Substances. A. Intermolecular Forces 1.... Course Hero Intermolecular Forces In Bromine The four key intermolecular forces are as follows: Compare the relative strengths of intermolecular forces; All of the attractive forces between. Identify the types of intermolecular forces experienced by specific molecules based on their structures; That is, they arise from the interaction between positively and negatively charged species. Let’s look at them individually, from strongest to weakest. Using a flowchart. Intermolecular Forces In Bromine.
From www.numerade.com
SOLVED What kind of intermolecular forces act between a carbon tetrachloride molecule and a Intermolecular Forces In Bromine Intermolecular forces are electrostatic in nature; Identify the types of intermolecular forces experienced by specific molecules based on their structures; The four key intermolecular forces are as follows: That is, they arise from the interaction between positively and negatively charged species. All of the attractive forces between. Distinguish between the following three types of intermolecular forces: Identify the different types. Intermolecular Forces In Bromine.
From ecampusontario.pressbooks.pub
13.1 Intermolecular Forces Enhanced Introductory College Chemistry Intermolecular Forces In Bromine The four key intermolecular forces are as follows: All of the attractive forces between. Like covalent and ionic bonds, intermolecular. Distinguish between the following three types of intermolecular forces: Compare the relative strengths of intermolecular forces; Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. In this video we’ll identify the intermolecular forces for br2 (diatomic. Intermolecular Forces In Bromine.
From www.bartleby.com
Answered Decide which intermolecular forces act… bartleby Intermolecular Forces In Bromine Compare the relative strengths of intermolecular forces; Using a flowchart to guide. Distinguish between the following three types of intermolecular forces: The extent to which one substance will dissolve in another is determined by several factors, including the types and relative strengths of. Identify the different types of intermolecular forces. That is, they arise from the interaction between positively and. Intermolecular Forces In Bromine.
From www.numerade.com
SOLVEDAt room temperature, bromine (Br2) is a corrosive red liquid, whereas iodine (I2) is a Intermolecular Forces In Bromine Compare the relative strengths of intermolecular forces; Intermolecular forces are electrostatic in nature; All of the attractive forces between. Relate the physical properties of a substance to the strength of attractive forces. Distinguish between the following three types of intermolecular forces: That is, they arise from the interaction between positively and negatively charged species. Like covalent and ionic bonds, intermolecular.. Intermolecular Forces In Bromine.
From sciencenotes.org
Intermolecular Forces in Chemistry Intermolecular Forces In Bromine Compare the relative strengths of intermolecular forces; The four key intermolecular forces are as follows: Identify the types of intermolecular forces experienced by specific molecules based on their structures; Like covalent and ionic bonds, intermolecular. Distinguish between the following three types of intermolecular forces: The extent to which one substance will dissolve in another is determined by several factors, including. Intermolecular Forces In Bromine.
From www.numerade.com
SOLVED Which answer best describes the strongest intermolecular force that exists between two Intermolecular Forces In Bromine Let’s look at them individually, from strongest to weakest. Identify the different types of intermolecular forces. Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. Identify the types of intermolecular forces experienced by specific molecules based on their structures; Compare the relative strengths of intermolecular forces; The extent to which one substance will dissolve in another. Intermolecular Forces In Bromine.
From www.gauthmath.com
Solved The boiling points of diatomic halogens are compared in the table. Boiling Points of Intermolecular Forces In Bromine Using a flowchart to guide. Compare the relative strengths of intermolecular forces; Like covalent and ionic bonds, intermolecular. In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/ molecular bromine). Identify the different types of intermolecular forces. Identify the types of intermolecular forces experienced by specific molecules based on their structures; That is, they arise from the interaction. Intermolecular Forces In Bromine.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID525098 Intermolecular Forces In Bromine All of the attractive forces between. Let’s look at them individually, from strongest to weakest. Compare the relative strengths of intermolecular forces; Distinguish between the following three types of intermolecular forces: The four key intermolecular forces are as follows: The extent to which one substance will dissolve in another is determined by several factors, including the types and relative strengths. Intermolecular Forces In Bromine.
From www.youtube.com
BrF Polar or Nonpolar (Bromine fluoride) YouTube Intermolecular Forces In Bromine Let’s look at them individually, from strongest to weakest. That is, they arise from the interaction between positively and negatively charged species. Like covalent and ionic bonds, intermolecular. Identify the different types of intermolecular forces. The extent to which one substance will dissolve in another is determined by several factors, including the types and relative strengths of. All of the. Intermolecular Forces In Bromine.
From www.numerade.com
In addition to dispersion forces, what intermolecular forces are present in a solution between Intermolecular Forces In Bromine Relate the physical properties of a substance to the strength of attractive forces. Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. Identify the different types of intermolecular forces. In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/ molecular bromine). Intermolecular forces are electrostatic in nature; That is, they arise from the interaction. Intermolecular Forces In Bromine.
From www.numerade.com
SOLVED Decide which intermolecular forces act between the molecules of each compound in the Intermolecular Forces In Bromine Relate the physical properties of a substance to the strength of attractive forces. The four key intermolecular forces are as follows: Intermolecular forces are electrostatic in nature; Like covalent and ionic bonds, intermolecular. Distinguish between the following three types of intermolecular forces: All of the attractive forces between. Identify the different types of intermolecular forces. In this video we’ll identify. Intermolecular Forces In Bromine.
From www.numerade.com
SOLVED Determine which intermolecular forces act between the molecules of each compound in the Intermolecular Forces In Bromine That is, they arise from the interaction between positively and negatively charged species. In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/ molecular bromine). Identify the types of intermolecular forces experienced by specific molecules based on their structures; Relate the physical properties of a substance to the strength of attractive forces. Using a flowchart to guide. The. Intermolecular Forces In Bromine.
From www.slideserve.com
PPT Comparing Intermolecular Force’s PowerPoint Presentation, free download ID6047087 Intermolecular Forces In Bromine Let’s look at them individually, from strongest to weakest. Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. The extent to which one substance will dissolve in another is determined by several factors, including the types and relative strengths of. That is, they arise from the interaction between positively and negatively charged species. Using a flowchart. Intermolecular Forces In Bromine.
From slideplayer.com
Intermolecular Forces Notes ppt download Intermolecular Forces In Bromine Compare the relative strengths of intermolecular forces; All of the attractive forces between. Identify the types of intermolecular forces experienced by specific molecules based on their structures; The extent to which one substance will dissolve in another is determined by several factors, including the types and relative strengths of. Distinguish between the following three types of intermolecular forces: In this. Intermolecular Forces In Bromine.
From slideplayer.com
Intermolecular Forces ppt download Intermolecular Forces In Bromine Identify the different types of intermolecular forces. Using a flowchart to guide. That is, they arise from the interaction between positively and negatively charged species. All of the attractive forces between. Identify the types of intermolecular forces experienced by specific molecules based on their structures; Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. Compare the. Intermolecular Forces In Bromine.
From www.youtube.com
1718 Intermolecular Forces YouTube Intermolecular Forces In Bromine Let’s look at them individually, from strongest to weakest. Identify the different types of intermolecular forces. Relate the physical properties of a substance to the strength of attractive forces. Compare the relative strengths of intermolecular forces; Distinguish between the following three types of intermolecular forces: Using a flowchart to guide. That is, they arise from the interaction between positively and. Intermolecular Forces In Bromine.
From www.clutchprep.com
Intermolecular Forces Chemistry Video Clutch Prep Intermolecular Forces In Bromine In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/ molecular bromine). All of the attractive forces between. The extent to which one substance will dissolve in another is determined by several factors, including the types and relative strengths of. Like covalent and ionic bonds, intermolecular. The four key intermolecular forces are as follows: Let’s look at them. Intermolecular Forces In Bromine.
From evulpo.com
Intermolecular forces types and examples Chemistry Explanation & Exercises evulpo Intermolecular Forces In Bromine The extent to which one substance will dissolve in another is determined by several factors, including the types and relative strengths of. Intermolecular forces are electrostatic in nature; Using a flowchart to guide. Distinguish between the following three types of intermolecular forces: That is, they arise from the interaction between positively and negatively charged species. Identify the different types of. Intermolecular Forces In Bromine.
From www.researchgate.net
(a) Intermolecular bromine/p interaction in the complex; (b) formation... Download Scientific Intermolecular Forces In Bromine That is, they arise from the interaction between positively and negatively charged species. Identify the different types of intermolecular forces. Let’s look at them individually, from strongest to weakest. Intermolecular forces are electrostatic in nature; Relate the physical properties of a substance to the strength of attractive forces. Using a flowchart to guide. The four key intermolecular forces are as. Intermolecular Forces In Bromine.
From slideplayer.com
Intermolecular Forces ppt download Intermolecular Forces In Bromine Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. The four key intermolecular forces are as follows: Intermolecular forces are electrostatic in nature; Identify the different types of intermolecular forces. Distinguish between the following three types of intermolecular forces: In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/ molecular bromine). Relate the physical. Intermolecular Forces In Bromine.
From www.numerade.com
SOLVED Decide which intermolecular forces act between the molecules of each compound in the Intermolecular Forces In Bromine The four key intermolecular forces are as follows: Like covalent and ionic bonds, intermolecular. That is, they arise from the interaction between positively and negatively charged species. The extent to which one substance will dissolve in another is determined by several factors, including the types and relative strengths of. In this video we’ll identify the intermolecular forces for br2 (diatomic. Intermolecular Forces In Bromine.
From slideplayer.com
Determine the Bond Type (Electronegativities) ppt download Intermolecular Forces In Bromine Compare the relative strengths of intermolecular forces; Relate the physical properties of a substance to the strength of attractive forces. That is, they arise from the interaction between positively and negatively charged species. Identify the different types of intermolecular forces. Intermolecular forces are electrostatic in nature; Distinguish between the following three types of intermolecular forces: Identify the types of intermolecular. Intermolecular Forces In Bromine.
From www.numerade.com
In addition to dispersion forces, what intermolecular forces are present in a solution between Intermolecular Forces In Bromine Let’s look at them individually, from strongest to weakest. Intermolecular forces are electrostatic in nature; Distinguish between the following three types of intermolecular forces: Using a flowchart to guide. The four key intermolecular forces are as follows: Like covalent and ionic bonds, intermolecular. Identify the different types of intermolecular forces. Relate the physical properties of a substance to the strength. Intermolecular Forces In Bromine.
From www.coursehero.com
[Solved] a.) Chlorine and Bromine are both diatomic. Explain the difference... Course Hero Intermolecular Forces In Bromine In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/ molecular bromine). All of the attractive forces between. The four key intermolecular forces are as follows: Distinguish between the following three types of intermolecular forces: The extent to which one substance will dissolve in another is determined by several factors, including the types and relative strengths of. Relate. Intermolecular Forces In Bromine.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation ID542353 Intermolecular Forces In Bromine Identify the types of intermolecular forces experienced by specific molecules based on their structures; Using a flowchart to guide. The four key intermolecular forces are as follows: Like covalent and ionic bonds, intermolecular. Compare the relative strengths of intermolecular forces; That is, they arise from the interaction between positively and negatively charged species. Relate the physical properties of a substance. Intermolecular Forces In Bromine.
From users.highland.edu
Intermolecular Forces Intermolecular Forces In Bromine Like covalent and ionic bonds, intermolecular. Intermolecular forces are electrostatic in nature; All of the attractive forces between. Identify the different types of intermolecular forces. The extent to which one substance will dissolve in another is determined by several factors, including the types and relative strengths of. Relate the physical properties of a substance to the strength of attractive forces.. Intermolecular Forces In Bromine.
From www.numerade.com
SOLVED Decide which Intermolecular forces act betwcen the molecules of each compound in the Intermolecular Forces In Bromine Distinguish between the following three types of intermolecular forces: Relate the physical properties of a substance to the strength of attractive forces. The extent to which one substance will dissolve in another is determined by several factors, including the types and relative strengths of. Using a flowchart to guide. Like covalent and ionic bonds, intermolecular. Identify the different types of. Intermolecular Forces In Bromine.
From www.slideserve.com
PPT Liquids, solids, & intermolecular forces PowerPoint Presentation ID1459744 Intermolecular Forces In Bromine Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. All of the attractive forces between. Using a flowchart to guide. Let’s look at them individually, from strongest to weakest. Identify the different types of intermolecular forces. The four key intermolecular forces are as follows: Like covalent and ionic bonds, intermolecular. Compare the relative strengths of intermolecular. Intermolecular Forces In Bromine.
From slideplayer.com
Determine the Bond Type (Electronegativities) ppt download Intermolecular Forces In Bromine Identify the different types of intermolecular forces. All of the attractive forces between. Intermolecular forces hold multiple molecules together and determine many of a substance’s properties. Let’s look at them individually, from strongest to weakest. Compare the relative strengths of intermolecular forces; In this video we’ll identify the intermolecular forces for br2 (diatomic bromine/ molecular bromine). Using a flowchart to. Intermolecular Forces In Bromine.