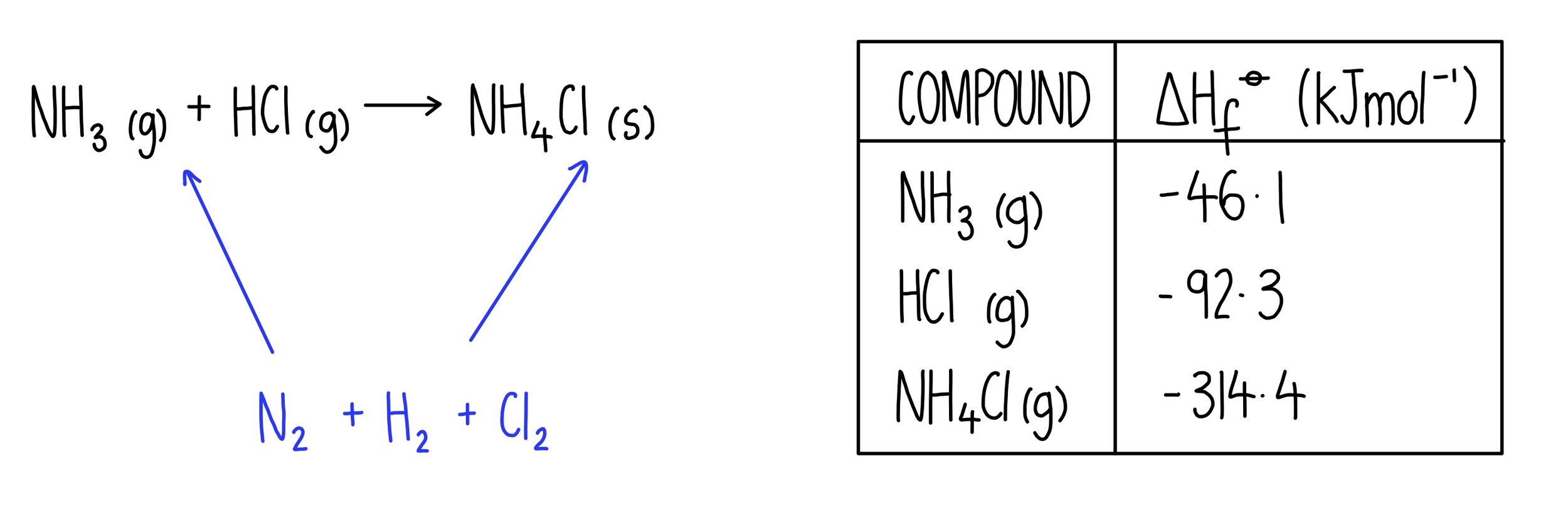
Standard Enthalpy Of Formation Equation Units . 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δhof = 46.0 kj. For example, the formation of 1 mol ammonia from h 2 and n 2 gases releases 46.0 kj heat: Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard heat of formation. A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with all pure substances present in their standard states (their most stable forms at 1 atm pressure and the temperature of the measurement). Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: The symbol for the standard enthalpy of formation is: The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. Δh f o all chemical reactions involve a change in enthalpy (defined as the heat produced or.
from www.thesciencehive.co.uk
A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard heat of formation. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. For example, the formation of 1 mol ammonia from h 2 and n 2 gases releases 46.0 kj heat: 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δhof = 46.0 kj. Δh f o all chemical reactions involve a change in enthalpy (defined as the heat produced or. A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with all pure substances present in their standard states (their most stable forms at 1 atm pressure and the temperature of the measurement).
Enthalpy Changes — the science hive
Standard Enthalpy Of Formation Equation Units 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with all pure substances present in their standard states (their most stable forms at 1 atm pressure and the temperature of the measurement). For example, the formation of 1 mol ammonia from h 2 and n 2 gases releases 46.0 kj heat: The symbol for the standard enthalpy of formation is: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard heat of formation. 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δhof = 46.0 kj. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. Δh f o all chemical reactions involve a change in enthalpy (defined as the heat produced or. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions:
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Enthalpy Of Formation Equation Units A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with all pure substances present in their standard states (their most stable forms at 1 atm pressure and the temperature of the measurement). The standard heat of formation. 193 rows the standard enthalpy of formation is measured in units of energy per amount. Standard Enthalpy Of Formation Equation Units.
From www.thesciencehive.co.uk
Enthalpy Changes — the science hive Standard Enthalpy Of Formation Equation Units Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δhof = 46.0 kj. The standard heat of formation. A pressure of 1 atm for gases and a concentration. Standard Enthalpy Of Formation Equation Units.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Equation Units Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The symbol for the standard enthalpy of formation is: 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δhof = 46.0 kj. Standard enthalpies of formation (\(δh^o_{f}\)) are. Standard Enthalpy Of Formation Equation Units.
From www.slideserve.com
PPT Chapter 15 Standard enthalpy change of a reaction PowerPoint Standard Enthalpy Of Formation Equation Units 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δhof = 46.0 kj. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: The symbol for the standard enthalpy of formation is: The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. For example,. Standard Enthalpy Of Formation Equation Units.
From mavink.com
Enthalpy Of Formation Equation Standard Enthalpy Of Formation Equation Units Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δhof = 46.0 kj. 193 rows the standard enthalpy of formation is measured in units of energy per amount. Standard Enthalpy Of Formation Equation Units.
From www.slideserve.com
PPT Ch. 15 Energy and Chemical Change PowerPoint Presentation, free Standard Enthalpy Of Formation Equation Units 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δhof = 46.0 kj. The symbol for the standard enthalpy of formation is: A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is a measure of the. Standard Enthalpy Of Formation Equation Units.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Equation Units Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δhof = 46.0 kj. 193 rows the standard enthalpy of formation is measured in units of energy per amount. Standard Enthalpy Of Formation Equation Units.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Equation Units A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g). Standard Enthalpy Of Formation Equation Units.
From mungfali.com
Standard Enthalpy Change Equation Standard Enthalpy Of Formation Equation Units For example, the formation of 1 mol ammonia from h 2 and n 2 gases releases 46.0 kj heat: The standard heat of formation. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δhof =. Standard Enthalpy Of Formation Equation Units.
From narodnatribuna.info
Enthalpy Equation Standard Enthalpy Of Formation Equation Units Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with all pure substances present in their standard states (their most stable forms. Standard Enthalpy Of Formation Equation Units.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Equation Units Δh f o all chemical reactions involve a change in enthalpy (defined as the heat produced or. For example, the formation of 1 mol ammonia from h 2 and n 2 gases releases 46.0 kj heat: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard.. Standard Enthalpy Of Formation Equation Units.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Equation Units A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. For example, the formation of 1 mol ammonia from h 2 and n 2 gases releases 46.0 kj heat: 193 rows the standard enthalpy of formation is measured in units of energy per amount. Standard Enthalpy Of Formation Equation Units.
From www.bartleby.com
Answered Using the standard enthalpies of… bartleby Standard Enthalpy Of Formation Equation Units Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. For example, the formation of 1 mol ammonia from h 2 and n 2 gases releases 46.0 kj heat: The standard enthalpy of formation, δh o f, is the enthalpy. Standard Enthalpy Of Formation Equation Units.
From www.toppr.com
Calculate the standard enthalpy of formation of CH3 OH from the Standard Enthalpy Of Formation Equation Units The symbol for the standard enthalpy of formation is: For example, the formation of 1 mol ammonia from h 2 and n 2 gases releases 46.0 kj heat: The standard heat of formation. A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with all pure substances present in their standard states (their. Standard Enthalpy Of Formation Equation Units.
From www.youtube.com
Writing Equations for Standard Enthalpy of Formation Examples YouTube Standard Enthalpy Of Formation Equation Units For example, the formation of 1 mol ammonia from h 2 and n 2 gases releases 46.0 kj heat: 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δhof = 46.0 kj. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: 193 rows the standard enthalpy of formation is measured in units of energy per amount of. Standard Enthalpy Of Formation Equation Units.
From www.toppr.com
Use the given standard enthalpies of formation (in kJ/mol) to determine Standard Enthalpy Of Formation Equation Units Δh f o all chemical reactions involve a change in enthalpy (defined as the heat produced or. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The symbol for the standard enthalpy of formation is: 193 rows the standard. Standard Enthalpy Of Formation Equation Units.
From www.youtube.com
Standard enthalpy of formation example question YouTube Standard Enthalpy Of Formation Equation Units The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows the standard enthalpy of formation is measured in units of. Standard Enthalpy Of Formation Equation Units.
From www.youtube.com
R1.2.3 / R1.2.4 Standard enthalpy change of formation (HL) YouTube Standard Enthalpy Of Formation Equation Units 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The symbol for the standard enthalpy of. Standard Enthalpy Of Formation Equation Units.
From www.chegg.com
Solved Use The Standard Enthalpies Of Formation In The Ta... Standard Enthalpy Of Formation Equation Units 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δhof = 46.0 kj. A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with all pure substances present. Standard Enthalpy Of Formation Equation Units.
From www.vrogue.co
Standard Enthalpy Of Reaction Units Slide Share vrogue.co Standard Enthalpy Of Formation Equation Units 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δhof = 46.0 kj. For example, the formation of 1 mol ammonia from h 2 and n 2 gases releases 46.0 kj heat: A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with all pure substances present in their standard states. Standard Enthalpy Of Formation Equation Units.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Equation Units The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δhof. Standard Enthalpy Of Formation Equation Units.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation Equation Units Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard heat of formation. For example, the formation of 1 mol ammonia from h 2 and n 2 gases releases 46.0 kj heat: The standard. Standard Enthalpy Of Formation Equation Units.
From worksheetdbblags.z13.web.core.windows.net
How To Find Standard Enthalpy Of Reaction Standard Enthalpy Of Formation Equation Units The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. Δh f o all chemical reactions involve a change in enthalpy (defined. Standard Enthalpy Of Formation Equation Units.
From studylib.net
2.5(a) Enthalpy Standard Enthalpy Of Formation Equation Units The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δhof = 46.0 kj. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: 193 rows the standard enthalpy of formation is measured in units. Standard Enthalpy Of Formation Equation Units.
From mavink.com
Standard Enthalpy Chart Standard Enthalpy Of Formation Equation Units For example, the formation of 1 mol ammonia from h 2 and n 2 gases releases 46.0 kj heat: A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is a measure of the energy released or consumed when. Standard Enthalpy Of Formation Equation Units.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Equation Units 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. Δh f o all chemical reactions involve a change in enthalpy (defined as the heat produced or. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance. Standard Enthalpy Of Formation Equation Units.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Enthalpy Of Formation Equation Units The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with all pure substances present in their standard states (their most stable forms at 1 atm pressure and the. Standard Enthalpy Of Formation Equation Units.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Enthalpy Of Formation Equation Units Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: For example, the formation of 1 mol ammonia from h 2 and n 2 gases releases 46.0 kj heat: The standard heat of formation. The symbol for the standard enthalpy of formation is: 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δhof = 46.0 kj. Δh f. Standard Enthalpy Of Formation Equation Units.
From www.tessshebaylo.com
Balance The Following Chemical Equation And Calculate Standard Enthalpy Standard Enthalpy Of Formation Equation Units Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 1.5h 2 (g) +. Standard Enthalpy Of Formation Equation Units.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in Standard Enthalpy Of Formation Equation Units The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δhof = 46.0 kj. Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: Standard enthalpy of formation (or heat of formation), δhof , is. Standard Enthalpy Of Formation Equation Units.
From www.slideshare.net
Tang 03 enthalpy of formation and combustion Standard Enthalpy Of Formation Equation Units Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: The symbol for the standard enthalpy of formation is: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A pressure of 1 atm for gases and a concentration of 1 m for species in solution,. Standard Enthalpy Of Formation Equation Units.
From www.chegg.com
Solved 13. The standard enthalpies of formation for several Standard Enthalpy Of Formation Equation Units Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δhof = 46.0 kj. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. For example, the formation of 1 mol ammonia from h 2. Standard Enthalpy Of Formation Equation Units.
From www.slideserve.com
PPT Free enthalpy Gibbs free energy PowerPoint Presentation, free Standard Enthalpy Of Formation Equation Units The standard heat of formation. Δh f o all chemical reactions involve a change in enthalpy (defined as the heat produced or. 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δhof = 46.0 kj. The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard. Standard Enthalpy Of Formation Equation Units.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation Equation Units Standard enthalpies of formation (\(δh^o_{f}\)) are determined under standard conditions: A pressure of 1 atm for gases and a concentration of 1 m for species in solution, with all pure substances present in their standard states (their most stable forms at 1 atm pressure and the temperature of the measurement). The standard enthalpy of formation is a measure of the. Standard Enthalpy Of Formation Equation Units.
From scienceinfo.com
Enthalpy Introduction, Calculation, Enthalpy change, Importance Standard Enthalpy Of Formation Equation Units The standard heat of formation. The symbol for the standard enthalpy of formation is: For example, the formation of 1 mol ammonia from h 2 and n 2 gases releases 46.0 kj heat: The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. A pressure of. Standard Enthalpy Of Formation Equation Units.