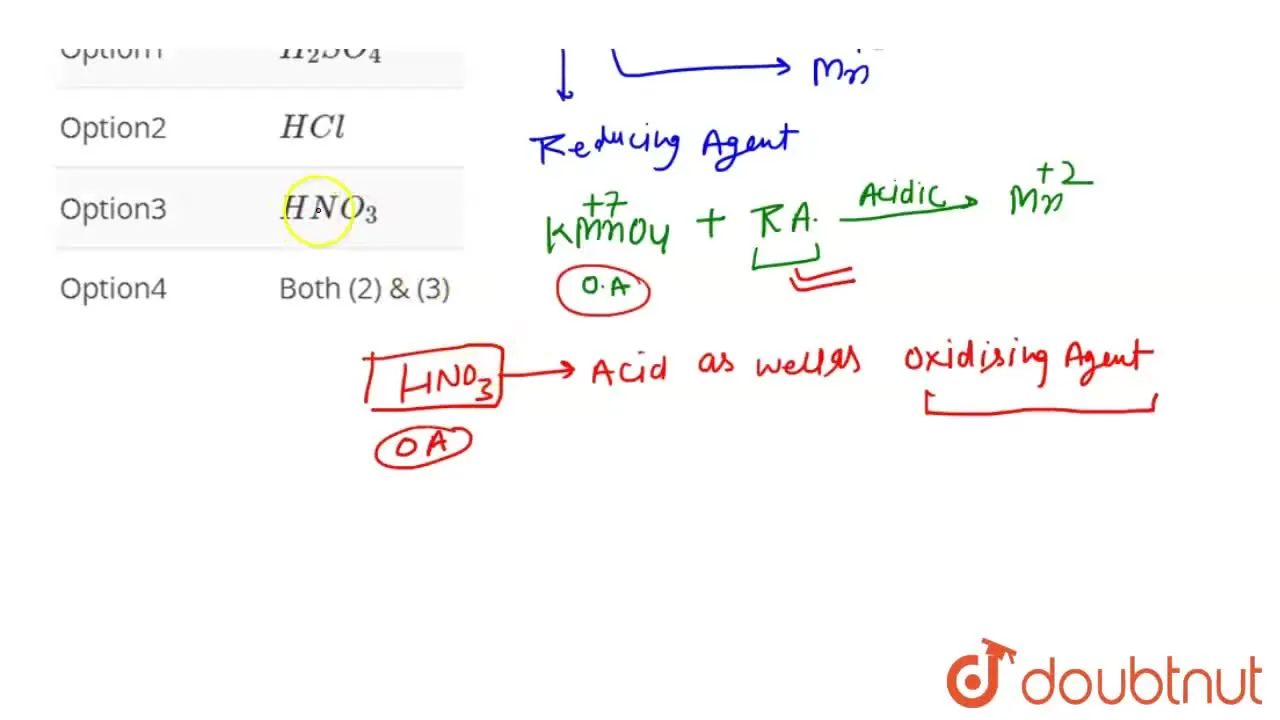
Titration Of Kmno4 In Acidic Medium . The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. The oxidising power of kmno4 depends on the medium: Kmno 4 acts as a good oxidising agent in acidic medium. This experiment involves the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4), which is an example of redox titration. The oxidising action of kmno in the acidic medium can be represented by the following. If acid is not used kmno 4 may be oxidised to mno 2 giving a brown precipitate. Kmno4 needs to be standardized before titration as it is not a primary standard. Therefore, the number of electrons transferred from oxidized species per mole $\ce{kmno4}$ (5 electrons) is the largest, and the least amount of $\ce{kmno4}$ is needed for.
from www.doubtnut.com
Kmno 4 acts as a good oxidising agent in acidic medium. This experiment involves the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4), which is an example of redox titration. If acid is not used kmno 4 may be oxidised to mno 2 giving a brown precipitate. Kmno4 needs to be standardized before titration as it is not a primary standard. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. The oxidising action of kmno in the acidic medium can be represented by the following. The oxidising power of kmno4 depends on the medium: Therefore, the number of electrons transferred from oxidized species per mole $\ce{kmno4}$ (5 electrons) is the largest, and the least amount of $\ce{kmno4}$ is needed for.
Titration of KMnO4 in acidic medium is not satisfactory in presence of
Titration Of Kmno4 In Acidic Medium The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Kmno4 needs to be standardized before titration as it is not a primary standard. Therefore, the number of electrons transferred from oxidized species per mole $\ce{kmno4}$ (5 electrons) is the largest, and the least amount of $\ce{kmno4}$ is needed for. The oxidising power of kmno4 depends on the medium: This experiment involves the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4), which is an example of redox titration. Kmno 4 acts as a good oxidising agent in acidic medium. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. If acid is not used kmno 4 may be oxidised to mno 2 giving a brown precipitate. The oxidising action of kmno in the acidic medium can be represented by the following.
From www.doubtnut.com
Titration of KMnO4 in acidic medium is not satisfactory in presence of Titration Of Kmno4 In Acidic Medium The oxidising power of kmno4 depends on the medium: Kmno 4 acts as a good oxidising agent in acidic medium. The oxidising action of kmno in the acidic medium can be represented by the following. If acid is not used kmno 4 may be oxidised to mno 2 giving a brown precipitate. This experiment involves the titration of potassium permanganate. Titration Of Kmno4 In Acidic Medium.
From www.chegg.com
Solved Sodium oxalate can be titrated with KMnO4 under Titration Of Kmno4 In Acidic Medium This experiment involves the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4), which is an example of redox titration. The oxidising power of kmno4 depends on the medium: If acid is not used kmno 4 may be oxidised to mno 2 giving a brown precipitate. Kmno4 needs to be standardized before titration as. Titration Of Kmno4 In Acidic Medium.
From www.doubtnut.com
Titration of KMnO4 in acidic medium is not satisfactory in presence of Titration Of Kmno4 In Acidic Medium This experiment involves the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4), which is an example of redox titration. The oxidising power of kmno4 depends on the medium: The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. If acid is. Titration Of Kmno4 In Acidic Medium.
From byjus.com
Find the number of moles of H2O2 required to completely react with Titration Of Kmno4 In Acidic Medium If acid is not used kmno 4 may be oxidised to mno 2 giving a brown precipitate. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. This experiment involves the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4), which is. Titration Of Kmno4 In Acidic Medium.
From chemistrypubs.com
Titration of Oxalic Acid with KMnO4 Chemistrupubs Titration Of Kmno4 In Acidic Medium Kmno4 needs to be standardized before titration as it is not a primary standard. The oxidising power of kmno4 depends on the medium: Kmno 4 acts as a good oxidising agent in acidic medium. The oxidising action of kmno in the acidic medium can be represented by the following. Therefore, the number of electrons transferred from oxidized species per mole. Titration Of Kmno4 In Acidic Medium.
From www.youtube.com
How to Prepare 1N or 0.1N KMnO4? Acidic, Basic and Neutral Medium Titration Of Kmno4 In Acidic Medium This experiment involves the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4), which is an example of redox titration. Therefore, the number of electrons transferred from oxidized species per mole $\ce{kmno4}$ (5 electrons) is the largest, and the least amount of $\ce{kmno4}$ is needed for. The titration of potassium permanganate (kmno 4) against. Titration Of Kmno4 In Acidic Medium.
From byjus.com
5.The number of moles of ferrous oxalate oxidised by one mole of kmno4 Titration Of Kmno4 In Acidic Medium The oxidising power of kmno4 depends on the medium: This experiment involves the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4), which is an example of redox titration. If acid is not used kmno 4 may be oxidised to mno 2 giving a brown precipitate. Therefore, the number of electrons transferred from oxidized. Titration Of Kmno4 In Acidic Medium.
From toppr.com
How many electrons are involved in the reduction of KMnO4 in acidic medium? Titration Of Kmno4 In Acidic Medium This experiment involves the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4), which is an example of redox titration. Therefore, the number of electrons transferred from oxidized species per mole $\ce{kmno4}$ (5 electrons) is the largest, and the least amount of $\ce{kmno4}$ is needed for. The oxidising action of kmno in the acidic. Titration Of Kmno4 In Acidic Medium.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Titration Of Kmno4 In Acidic Medium The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Kmno4 needs to be standardized before titration as it is not a primary standard. Kmno 4 acts as a good oxidising agent in acidic medium. The oxidising action of kmno in the acidic medium can be represented by. Titration Of Kmno4 In Acidic Medium.
From www.mutualgreget.com
What Is The Molar Mass Of Kmno4 mutualgreget Titration Of Kmno4 In Acidic Medium Kmno 4 acts as a good oxidising agent in acidic medium. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. If acid is not used kmno 4 may be oxidised to mno 2 giving a brown precipitate. Therefore, the number of electrons transferred from oxidized species per. Titration Of Kmno4 In Acidic Medium.
From byjus.com
8 gm sample of KMnO4 is rected in acidic medium with 100ml of 10 valume Titration Of Kmno4 In Acidic Medium The oxidising power of kmno4 depends on the medium: Kmno 4 acts as a good oxidising agent in acidic medium. If acid is not used kmno 4 may be oxidised to mno 2 giving a brown precipitate. Therefore, the number of electrons transferred from oxidized species per mole $\ce{kmno4}$ (5 electrons) is the largest, and the least amount of $\ce{kmno4}$. Titration Of Kmno4 In Acidic Medium.
From byjus.com
The weight of KMnO4 that can oxidise 100 ml of 0.2 M oxalic acid in Titration Of Kmno4 In Acidic Medium If acid is not used kmno 4 may be oxidised to mno 2 giving a brown precipitate. Therefore, the number of electrons transferred from oxidized species per mole $\ce{kmno4}$ (5 electrons) is the largest, and the least amount of $\ce{kmno4}$ is needed for. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is. Titration Of Kmno4 In Acidic Medium.
From www.youtube.com
KMnO4 Reactions in Acidic Medium Class 12th How to Balance Titration Of Kmno4 In Acidic Medium This experiment involves the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4), which is an example of redox titration. The oxidising action of kmno in the acidic medium can be represented by the following. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example. Titration Of Kmno4 In Acidic Medium.
From www.toppr.com
It was observed that in the initial additions of KMnO4 in the titration Titration Of Kmno4 In Acidic Medium Kmno 4 acts as a good oxidising agent in acidic medium. The oxidising action of kmno in the acidic medium can be represented by the following. Therefore, the number of electrons transferred from oxidized species per mole $\ce{kmno4}$ (5 electrons) is the largest, and the least amount of $\ce{kmno4}$ is needed for. The titration of potassium permanganate (kmno 4) against. Titration Of Kmno4 In Acidic Medium.
From www.youtube.com
titration of kmno4 with mohr's salt class 12 determine the Titration Of Kmno4 In Acidic Medium If acid is not used kmno 4 may be oxidised to mno 2 giving a brown precipitate. This experiment involves the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4), which is an example of redox titration. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is. Titration Of Kmno4 In Acidic Medium.
From byjus.com
71 Molecular weight of KMnO4 in acidic medium and neutral medium will Titration Of Kmno4 In Acidic Medium Therefore, the number of electrons transferred from oxidized species per mole $\ce{kmno4}$ (5 electrons) is the largest, and the least amount of $\ce{kmno4}$ is needed for. The oxidising power of kmno4 depends on the medium: This experiment involves the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4), which is an example of redox. Titration Of Kmno4 In Acidic Medium.
From www.numerade.com
SOLVED KMnO4, can be standardized in Potassium permanganate, acidic Titration Of Kmno4 In Acidic Medium The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Therefore, the number of electrons transferred from oxidized species per mole $\ce{kmno4}$ (5 electrons) is the largest, and the least amount of $\ce{kmno4}$ is needed for. The oxidising power of kmno4 depends on the medium: The oxidising action. Titration Of Kmno4 In Acidic Medium.
From www.youtube.com
Why KMnO4 is a strong oxidizing agent in acidic medium?? YouTube Titration Of Kmno4 In Acidic Medium The oxidising power of kmno4 depends on the medium: The oxidising action of kmno in the acidic medium can be represented by the following. Therefore, the number of electrons transferred from oxidized species per mole $\ce{kmno4}$ (5 electrons) is the largest, and the least amount of $\ce{kmno4}$ is needed for. The titration of potassium permanganate (kmno 4) against oxalic acid. Titration Of Kmno4 In Acidic Medium.
From byjus.com
Calculate the number of moles of KMNO4 required to oxidise one mole of Titration Of Kmno4 In Acidic Medium Therefore, the number of electrons transferred from oxidized species per mole $\ce{kmno4}$ (5 electrons) is the largest, and the least amount of $\ce{kmno4}$ is needed for. The oxidising power of kmno4 depends on the medium: Kmno 4 acts as a good oxidising agent in acidic medium. If acid is not used kmno 4 may be oxidised to mno 2 giving. Titration Of Kmno4 In Acidic Medium.
From www.toppr.com
It was observed that in the initial additions of KMnO4 in the titration Titration Of Kmno4 In Acidic Medium The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. This experiment involves the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4), which is an example of redox titration. Therefore, the number of electrons transferred from oxidized species per mole $\ce{kmno4}$. Titration Of Kmno4 In Acidic Medium.
From www.toppr.com
Explain why does color of KMnO4 disappear when oxalic acid is added to Titration Of Kmno4 In Acidic Medium The oxidising power of kmno4 depends on the medium: This experiment involves the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4), which is an example of redox titration. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Kmno 4 acts. Titration Of Kmno4 In Acidic Medium.
From www.numerade.com
SOLVEDRedox Titrationi Acidic Medium Puch Slider Upto Add Volume of Titration Of Kmno4 In Acidic Medium The oxidising power of kmno4 depends on the medium: This experiment involves the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4), which is an example of redox titration. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Therefore, the number. Titration Of Kmno4 In Acidic Medium.
From www.youtube.com
KMnO4 in Acid, Base and Neutral Medium Oxidation State Equivalent Titration Of Kmno4 In Acidic Medium The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Therefore, the number of electrons transferred from oxidized species per mole $\ce{kmno4}$ (5 electrons) is the largest, and the least amount of $\ce{kmno4}$ is needed for. Kmno4 needs to be standardized before titration as it is not a. Titration Of Kmno4 In Acidic Medium.
From www.toppr.com
How many moles of KMnO4 are required to oxidize one mole of SnCl2 in Titration Of Kmno4 In Acidic Medium Kmno4 needs to be standardized before titration as it is not a primary standard. The oxidising power of kmno4 depends on the medium: Kmno 4 acts as a good oxidising agent in acidic medium. If acid is not used kmno 4 may be oxidised to mno 2 giving a brown precipitate. The titration of potassium permanganate (kmno 4) against oxalic. Titration Of Kmno4 In Acidic Medium.
From byjus.com
the moles of KMnO4 require to oxidise 100ml of 1volume H2O2 in acidic Titration Of Kmno4 In Acidic Medium This experiment involves the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4), which is an example of redox titration. Kmno4 needs to be standardized before titration as it is not a primary standard. The oxidising action of kmno in the acidic medium can be represented by the following. Therefore, the number of electrons. Titration Of Kmno4 In Acidic Medium.
From www.youtube.com
Titration KMnO4 Vs Mohr's Salt Full Experiment + Calculation YouTube Titration Of Kmno4 In Acidic Medium Kmno4 needs to be standardized before titration as it is not a primary standard. Therefore, the number of electrons transferred from oxidized species per mole $\ce{kmno4}$ (5 electrons) is the largest, and the least amount of $\ce{kmno4}$ is needed for. If acid is not used kmno 4 may be oxidised to mno 2 giving a brown precipitate. Kmno 4 acts. Titration Of Kmno4 In Acidic Medium.
From www.youtube.com
How to Balance Acidic KMnO4 + H2S( Half Reaction Method in acidic Titration Of Kmno4 In Acidic Medium Kmno 4 acts as a good oxidising agent in acidic medium. If acid is not used kmno 4 may be oxidised to mno 2 giving a brown precipitate. Therefore, the number of electrons transferred from oxidized species per mole $\ce{kmno4}$ (5 electrons) is the largest, and the least amount of $\ce{kmno4}$ is needed for. This experiment involves the titration of. Titration Of Kmno4 In Acidic Medium.
From byjus.com
One mole of KMnO4 is used for complete oxidation of FeSO4, FeC2O4 and Titration Of Kmno4 In Acidic Medium The oxidising power of kmno4 depends on the medium: Therefore, the number of electrons transferred from oxidized species per mole $\ce{kmno4}$ (5 electrons) is the largest, and the least amount of $\ce{kmno4}$ is needed for. If acid is not used kmno 4 may be oxidised to mno 2 giving a brown precipitate. This experiment involves the titration of potassium permanganate. Titration Of Kmno4 In Acidic Medium.
From mavink.com
H Kmno4 Titration Of Kmno4 In Acidic Medium The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Therefore, the number of electrons transferred from oxidized species per mole $\ce{kmno4}$ (5 electrons) is the largest, and the least amount of $\ce{kmno4}$ is needed for. Kmno 4 acts as a good oxidising agent in acidic medium. If. Titration Of Kmno4 In Acidic Medium.
From byjus.com
0.2 g sample of H2O2 required 10 ml of 1N KMnO4 for complete reaction Titration Of Kmno4 In Acidic Medium If acid is not used kmno 4 may be oxidised to mno 2 giving a brown precipitate. Kmno 4 acts as a good oxidising agent in acidic medium. Therefore, the number of electrons transferred from oxidized species per mole $\ce{kmno4}$ (5 electrons) is the largest, and the least amount of $\ce{kmno4}$ is needed for. Kmno4 needs to be standardized before. Titration Of Kmno4 In Acidic Medium.
From www.toppr.com
KMnO4 acts as an oxidizing agent in acidic medium. The number of moles Titration Of Kmno4 In Acidic Medium Kmno 4 acts as a good oxidising agent in acidic medium. This experiment involves the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4), which is an example of redox titration. Kmno4 needs to be standardized before titration as it is not a primary standard. The titration of potassium permanganate (kmno 4) against oxalic. Titration Of Kmno4 In Acidic Medium.
From byjus.com
Increasing order of oxidising power of KMnO4 in different medium(acidic Titration Of Kmno4 In Acidic Medium The oxidising power of kmno4 depends on the medium: The oxidising action of kmno in the acidic medium can be represented by the following. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Kmno4 needs to be standardized before titration as it is not a primary standard.. Titration Of Kmno4 In Acidic Medium.
From byjus.com
100 mL of 1 M KMnO4 oxidised 100 mL H2O2 in acidic. Calculate the Titration Of Kmno4 In Acidic Medium Kmno 4 acts as a good oxidising agent in acidic medium. The oxidising action of kmno in the acidic medium can be represented by the following. If acid is not used kmno 4 may be oxidised to mno 2 giving a brown precipitate. Therefore, the number of electrons transferred from oxidized species per mole $\ce{kmno4}$ (5 electrons) is the largest,. Titration Of Kmno4 In Acidic Medium.
From byjus.com
38. Equivalent weight of KMno4 in basic medium Titration Of Kmno4 In Acidic Medium The oxidising power of kmno4 depends on the medium: Kmno4 needs to be standardized before titration as it is not a primary standard. This experiment involves the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4), which is an example of redox titration. The titration of potassium permanganate (kmno 4) against oxalic acid (c. Titration Of Kmno4 In Acidic Medium.
From chemistrypubs.com
Titration of Oxalic Acid with KMnO4 Chemistrupubs Titration Of Kmno4 In Acidic Medium The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Kmno 4 acts as a good oxidising agent in acidic medium. The oxidising power of kmno4 depends on the medium: Kmno4 needs to be standardized before titration as it is not a primary standard. If acid is not. Titration Of Kmno4 In Acidic Medium.