K Value Kinetics . Because products are in the numerator of the. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The values of k shown in table 15.2.2, for example, vary by 60 orders of magnitude. From the michaelis menten kinetic equation, we have many. The initial rates and the rate equation; The rate constant, k, gives a direct measure of the relative reaction rate. Equation • a typical rate equation may like this r = k [a] [b]2 • it shows how rate is affected by the concentrations of reactants [a] and [b] • the rate. A very small value for the rate constant equates to a very slow reaction in. Therefore, \(k_m\) is equal to the concentration of the substrate when the rate is half of the maximum velocity. The rate constant (k) of a reaction can be calculated using:
from www.slideserve.com
From the michaelis menten kinetic equation, we have many. A very small value for the rate constant equates to a very slow reaction in. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. Therefore, \(k_m\) is equal to the concentration of the substrate when the rate is half of the maximum velocity. Because products are in the numerator of the. Equation • a typical rate equation may like this r = k [a] [b]2 • it shows how rate is affected by the concentrations of reactants [a] and [b] • the rate. The values of k shown in table 15.2.2, for example, vary by 60 orders of magnitude. The rate constant, k, gives a direct measure of the relative reaction rate. The initial rates and the rate equation; The rate constant (k) of a reaction can be calculated using:
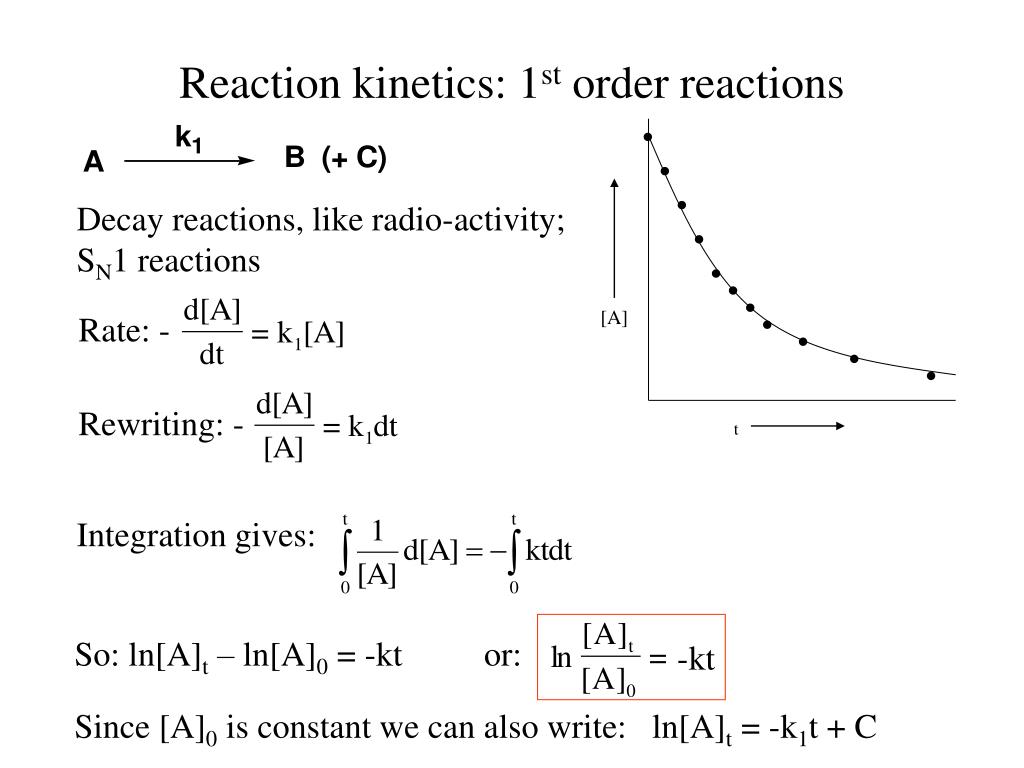
PPT Reaction 1 st order reactions PowerPoint Presentation
K Value Kinetics The rate constant (k) of a reaction can be calculated using: The rate constant, k, gives a direct measure of the relative reaction rate. Equation • a typical rate equation may like this r = k [a] [b]2 • it shows how rate is affected by the concentrations of reactants [a] and [b] • the rate. Therefore, \(k_m\) is equal to the concentration of the substrate when the rate is half of the maximum velocity. From the michaelis menten kinetic equation, we have many. The rate constant (k) of a reaction can be calculated using: The initial rates and the rate equation; A very small value for the rate constant equates to a very slow reaction in. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. Because products are in the numerator of the. The values of k shown in table 15.2.2, for example, vary by 60 orders of magnitude.
From www.graphpad.com
GraphPad Prism 9 Curve Fitting Guide Equation Determine kcat K Value Kinetics From the michaelis menten kinetic equation, we have many. Therefore, \(k_m\) is equal to the concentration of the substrate when the rate is half of the maximum velocity. Equation • a typical rate equation may like this r = k [a] [b]2 • it shows how rate is affected by the concentrations of reactants [a] and [b] • the rate.. K Value Kinetics.
From www.slideserve.com
PPT Reaction 1 st order reactions PowerPoint Presentation K Value Kinetics Because products are in the numerator of the. The rate constant (k) of a reaction can be calculated using: The rate constant, k, gives a direct measure of the relative reaction rate. Equation • a typical rate equation may like this r = k [a] [b]2 • it shows how rate is affected by the concentrations of reactants [a] and. K Value Kinetics.
From pametno21.blogspot.com
K Constant Formula Chemistry pametno K Value Kinetics The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. Because products are in the numerator of the. The initial rates and the rate equation; Equation • a typical rate equation may like this r = k [a] [b]2 • it shows how rate is affected by the. K Value Kinetics.
From www.youtube.com
For a reaction, the values of rate constant k at two K Value Kinetics The initial rates and the rate equation; Equation • a typical rate equation may like this r = k [a] [b]2 • it shows how rate is affected by the concentrations of reactants [a] and [b] • the rate. The rate constant, k, gives a direct measure of the relative reaction rate. The rate constant (k) of a reaction can. K Value Kinetics.
From www.slideserve.com
PPT 513341 Biochemistry I Chapter 7 ENZYME PowerPoint K Value Kinetics The rate constant (k) of a reaction can be calculated using: A very small value for the rate constant equates to a very slow reaction in. The values of k shown in table 15.2.2, for example, vary by 60 orders of magnitude. Because products are in the numerator of the. The rate constant, k, is a number that connects the. K Value Kinetics.
From www.britannica.com
Chemical Definition, Equations, & Facts Britannica K Value Kinetics From the michaelis menten kinetic equation, we have many. The rate constant, k, gives a direct measure of the relative reaction rate. A very small value for the rate constant equates to a very slow reaction in. Therefore, \(k_m\) is equal to the concentration of the substrate when the rate is half of the maximum velocity. The rate constant, k,. K Value Kinetics.
From www.researchgate.net
Firstorder reaction rate constants (k obs ) and initial reaction rates K Value Kinetics A very small value for the rate constant equates to a very slow reaction in. From the michaelis menten kinetic equation, we have many. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. Because products are in the numerator of the. Therefore, \(k_m\) is equal to the. K Value Kinetics.
From www.slideserve.com
PPT Chapter 6.3 Enzyme PowerPoint Presentation, free K Value Kinetics The rate constant, k, gives a direct measure of the relative reaction rate. The rate constant (k) of a reaction can be calculated using: The values of k shown in table 15.2.2, for example, vary by 60 orders of magnitude. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of. K Value Kinetics.
From www.slideshare.net
Chemical K Value Kinetics Equation • a typical rate equation may like this r = k [a] [b]2 • it shows how rate is affected by the concentrations of reactants [a] and [b] • the rate. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. Therefore, \(k_m\) is equal to the. K Value Kinetics.
From www.biologyonline.com
Firstorder Definition and Examples Biology Online Dictionary K Value Kinetics Equation • a typical rate equation may like this r = k [a] [b]2 • it shows how rate is affected by the concentrations of reactants [a] and [b] • the rate. From the michaelis menten kinetic equation, we have many. A very small value for the rate constant equates to a very slow reaction in. The values of k. K Value Kinetics.
From www.youtube.com
Plotting data for a firstorder reaction Chemistry Khan K Value Kinetics The initial rates and the rate equation; The values of k shown in table 15.2.2, for example, vary by 60 orders of magnitude. The rate constant, k, gives a direct measure of the relative reaction rate. From the michaelis menten kinetic equation, we have many. Because products are in the numerator of the. Therefore, \(k_m\) is equal to the concentration. K Value Kinetics.
From www.youtube.com
Integrated Rate Laws Zero, First, & Second Order Reactions Chemical K Value Kinetics A very small value for the rate constant equates to a very slow reaction in. The rate constant, k, gives a direct measure of the relative reaction rate. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The initial rates and the rate equation; Because products are. K Value Kinetics.
From www.youtube.com
What is the dissocation constant KD? (Part 1 of the KD video series K Value Kinetics From the michaelis menten kinetic equation, we have many. Therefore, \(k_m\) is equal to the concentration of the substrate when the rate is half of the maximum velocity. The rate constant, k, gives a direct measure of the relative reaction rate. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate. K Value Kinetics.
From www.youtube.com
Chemical Initial Rates Method YouTube K Value Kinetics Because products are in the numerator of the. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The values of k shown in table 15.2.2, for example, vary by 60 orders of magnitude. Equation • a typical rate equation may like this r = k [a] [b]2. K Value Kinetics.
From enzymkinetics.com
Enzyme BestCurvFit Software (EZFit, Perrella), K Value Kinetics Because products are in the numerator of the. A very small value for the rate constant equates to a very slow reaction in. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. From the michaelis menten kinetic equation, we have many. The initial rates and the rate. K Value Kinetics.
From www.youtube.com
B.7 Vmax and Km (HL) YouTube K Value Kinetics The values of k shown in table 15.2.2, for example, vary by 60 orders of magnitude. Therefore, \(k_m\) is equal to the concentration of the substrate when the rate is half of the maximum velocity. Equation • a typical rate equation may like this r = k [a] [b]2 • it shows how rate is affected by the concentrations of. K Value Kinetics.
From study.com
Solving for FirstOrder given HalfLife Chemistry K Value Kinetics The rate constant, k, gives a direct measure of the relative reaction rate. Therefore, \(k_m\) is equal to the concentration of the substrate when the rate is half of the maximum velocity. Equation • a typical rate equation may like this r = k [a] [b]2 • it shows how rate is affected by the concentrations of reactants [a] and. K Value Kinetics.
From www.slideserve.com
PPT 513341 Biochemistry I Chapter 7 ENZYME PowerPoint K Value Kinetics From the michaelis menten kinetic equation, we have many. Therefore, \(k_m\) is equal to the concentration of the substrate when the rate is half of the maximum velocity. The values of k shown in table 15.2.2, for example, vary by 60 orders of magnitude. Because products are in the numerator of the. A very small value for the rate constant. K Value Kinetics.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Reaction (5) and Mechanism K Value Kinetics The rate constant (k) of a reaction can be calculated using: The initial rates and the rate equation; The values of k shown in table 15.2.2, for example, vary by 60 orders of magnitude. From the michaelis menten kinetic equation, we have many. Therefore, \(k_m\) is equal to the concentration of the substrate when the rate is half of the. K Value Kinetics.
From www.youtube.com
Constants Km & Vmax YouTube K Value Kinetics The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The rate constant, k, gives a direct measure of the relative reaction rate. Equation • a typical rate equation may like this r = k [a] [b]2 • it shows how rate is affected by the concentrations of. K Value Kinetics.
From www.chegg.com
Solved Chemical , Rate Law, k Using experimented K Value Kinetics Because products are in the numerator of the. The rate constant, k, gives a direct measure of the relative reaction rate. The initial rates and the rate equation; From the michaelis menten kinetic equation, we have many. Therefore, \(k_m\) is equal to the concentration of the substrate when the rate is half of the maximum velocity. The rate constant, k,. K Value Kinetics.
From www.youtube.com
How To Determine The Units Of The Rate Constant K Chemical K Value Kinetics A very small value for the rate constant equates to a very slow reaction in. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. Because products are in the numerator of the. The rate constant (k) of a reaction can be calculated using: From the michaelis menten. K Value Kinetics.
From www.researchgate.net
result showing R 2 and k values for all models tested K Value Kinetics From the michaelis menten kinetic equation, we have many. The values of k shown in table 15.2.2, for example, vary by 60 orders of magnitude. The initial rates and the rate equation; The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. Because products are in the numerator. K Value Kinetics.
From www.slideshare.net
Chemical K Value Kinetics From the michaelis menten kinetic equation, we have many. A very small value for the rate constant equates to a very slow reaction in. Therefore, \(k_m\) is equal to the concentration of the substrate when the rate is half of the maximum velocity. The rate constant, k, gives a direct measure of the relative reaction rate. The values of k. K Value Kinetics.
From 2012books.lardbucket.org
Chemical K Value Kinetics The rate constant, k, gives a direct measure of the relative reaction rate. The values of k shown in table 15.2.2, for example, vary by 60 orders of magnitude. From the michaelis menten kinetic equation, we have many. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction.. K Value Kinetics.
From guruguruips.blogspot.com
Ki Kd Ec50 Guru IPS K Value Kinetics From the michaelis menten kinetic equation, we have many. The rate constant, k, gives a direct measure of the relative reaction rate. Therefore, \(k_m\) is equal to the concentration of the substrate when the rate is half of the maximum velocity. The values of k shown in table 15.2.2, for example, vary by 60 orders of magnitude. Because products are. K Value Kinetics.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID1477171 K Value Kinetics The rate constant, k, gives a direct measure of the relative reaction rate. A very small value for the rate constant equates to a very slow reaction in. The initial rates and the rate equation; Equation • a typical rate equation may like this r = k [a] [b]2 • it shows how rate is affected by the concentrations of. K Value Kinetics.
From www.numerade.com
SOLVED Enzyme Quick recap What happens at zero [S]? E + 5 ES K Value Kinetics Because products are in the numerator of the. From the michaelis menten kinetic equation, we have many. The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction. The rate constant, k, gives a direct measure of the relative reaction rate. The rate constant (k) of a reaction can. K Value Kinetics.
From chem.libretexts.org
Chapter 13.4 Using Graphs to Determine Rate Laws, Rate Constants and K Value Kinetics From the michaelis menten kinetic equation, we have many. A very small value for the rate constant equates to a very slow reaction in. The rate constant, k, gives a direct measure of the relative reaction rate. The values of k shown in table 15.2.2, for example, vary by 60 orders of magnitude. The rate constant (k) of a reaction. K Value Kinetics.
From www.slideserve.com
PPT 513341 Biochemistry I Chapter 7 ENZYME PowerPoint K Value Kinetics Because products are in the numerator of the. The values of k shown in table 15.2.2, for example, vary by 60 orders of magnitude. The rate constant (k) of a reaction can be calculated using: A very small value for the rate constant equates to a very slow reaction in. Equation • a typical rate equation may like this r. K Value Kinetics.
From www.researchgate.net
Value of k and potential N mineralization on firstorder K Value Kinetics The rate constant (k) of a reaction can be calculated using: The initial rates and the rate equation; A very small value for the rate constant equates to a very slow reaction in. From the michaelis menten kinetic equation, we have many. Therefore, \(k_m\) is equal to the concentration of the substrate when the rate is half of the maximum. K Value Kinetics.
From www.toppr.com
The graph of energy (K) of a body versus v K Value Kinetics Because products are in the numerator of the. The rate constant, k, gives a direct measure of the relative reaction rate. A very small value for the rate constant equates to a very slow reaction in. The initial rates and the rate equation; The rate constant, k, is a number that connects the concentration of reactants in a reaction to. K Value Kinetics.
From www.youtube.com
units of the rate constant k derivations YouTube K Value Kinetics Therefore, \(k_m\) is equal to the concentration of the substrate when the rate is half of the maximum velocity. The rate constant, k, gives a direct measure of the relative reaction rate. A very small value for the rate constant equates to a very slow reaction in. The initial rates and the rate equation; From the michaelis menten kinetic equation,. K Value Kinetics.
From wiki.whitson.com
Equilibrium Ratios whitson wiki K Value Kinetics Equation • a typical rate equation may like this r = k [a] [b]2 • it shows how rate is affected by the concentrations of reactants [a] and [b] • the rate. Therefore, \(k_m\) is equal to the concentration of the substrate when the rate is half of the maximum velocity. The values of k shown in table 15.2.2, for. K Value Kinetics.
From socratic.org
What happens as reactants get consumed while approaching a chemical K Value Kinetics Equation • a typical rate equation may like this r = k [a] [b]2 • it shows how rate is affected by the concentrations of reactants [a] and [b] • the rate. The rate constant (k) of a reaction can be calculated using: The rate constant, k, gives a direct measure of the relative reaction rate. The values of k. K Value Kinetics.