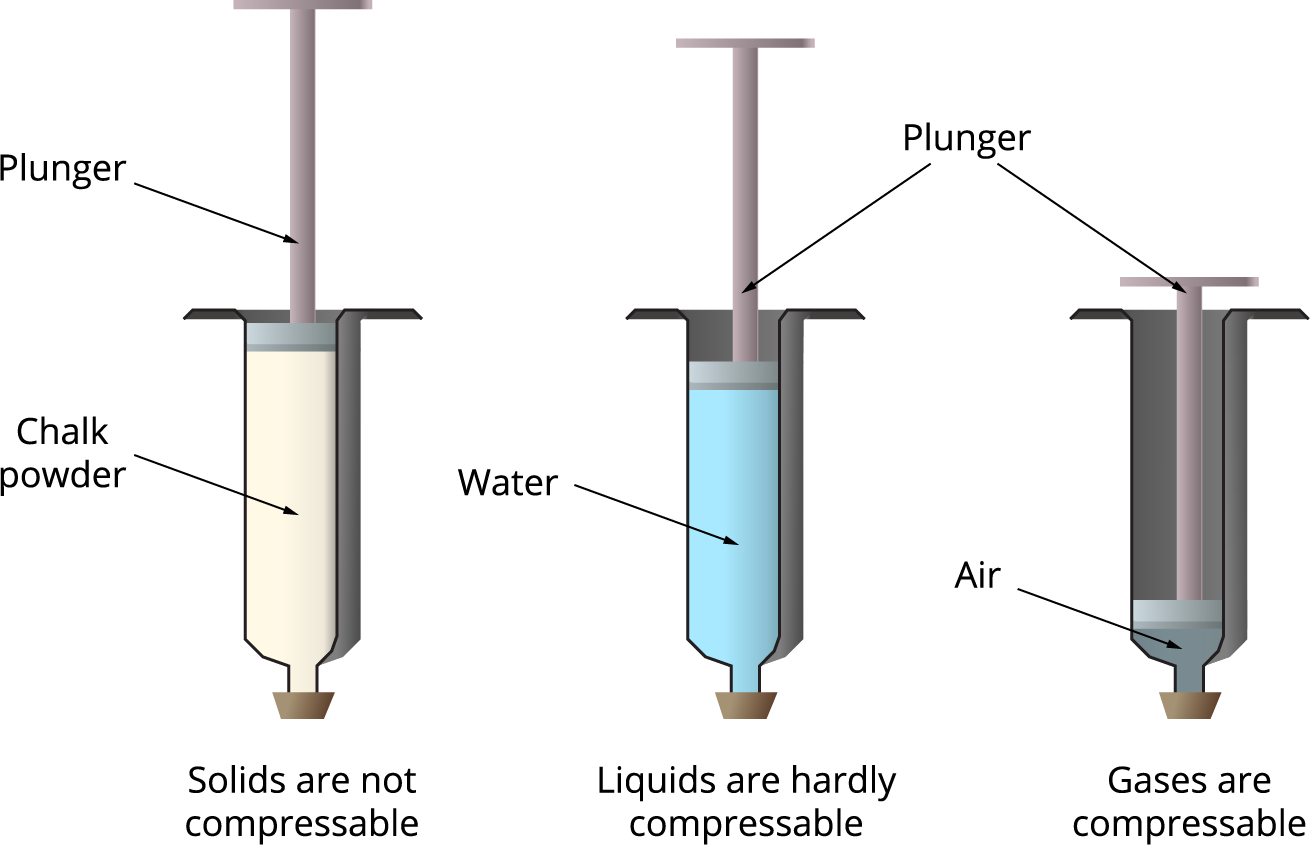
Solid Liquid Gas Compressibility . Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. Liquids can flow easily and assume the shape of their. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). Gases are highly compressible compared to solids and liquids due to the larger. Gases are compressible because most. And why they adopt the shape (but not the volume) of their containers. The thermal expansion of liquids; Solids maintain a fixed volume and shape and are not easily compressed. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Gases have a low density because their particles are very far apart and occupy any given space, leading to a greater volume relative to the number of particles. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it.
from www.yaclass.in
Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. And why they adopt the shape (but not the volume) of their containers. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Gases are compressible because most. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. Gases have a low density because their particles are very far apart and occupy any given space, leading to a greater volume relative to the number of particles. Gases are highly compressible compared to solids and liquids due to the larger. Liquids can flow easily and assume the shape of their. The thermal expansion of liquids;
Compressibility of solids, liquids and gases — lesson. Science State
Solid Liquid Gas Compressibility This model explains the higher density, greater order, and lower compressibility of liquids versus gases; And why they adopt the shape (but not the volume) of their containers. Gases are compressible because most. Gases have a low density because their particles are very far apart and occupy any given space, leading to a greater volume relative to the number of particles. Solids maintain a fixed volume and shape and are not easily compressed. Gases are highly compressible compared to solids and liquids due to the larger. The thermal expansion of liquids; This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Liquids can flow easily and assume the shape of their. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!).
From www.numerade.com
SOLVED Compare the compressibility of solids and liquids. Support your Solid Liquid Gas Compressibility Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Solids maintain a fixed volume and shape and are not easily compressed. Liquids can flow easily and assume the shape of their. Gases have a low density because. Solid Liquid Gas Compressibility.
From brainly.in
State the reason behind the observation recorded in the activity to Solid Liquid Gas Compressibility Liquids can flow easily and assume the shape of their. Gases are highly compressible compared to solids and liquids due to the larger. Gases are compressible because most. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. This model explains the higher density, greater order, and lower compressibility of liquids versus gases;. Solid Liquid Gas Compressibility.
From primaryleap.co.uk
Chemistry States Of Matter Level 1 activity for kids PrimaryLeap.co.uk Solid Liquid Gas Compressibility And why they adopt the shape (but not the volume) of their containers. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Gases are highly compressible compared to solids and liquids due to the larger. Solids maintain a fixed volume and shape and are not easily compressed. Gases are compressible because most.. Solid Liquid Gas Compressibility.
From www.pw.live
Class 9 chemistry Notes Matter is our surrounding STATES OF MATTER Solid Liquid Gas Compressibility Gases have a low density because their particles are very far apart and occupy any given space, leading to a greater volume relative to the number of particles. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). Gases are compressible because most. Solids maintain a fixed volume and shape and are not. Solid Liquid Gas Compressibility.
From www.slideshare.net
(Science) Matter Solid Liquid Gas Compressibility Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). The thermal expansion of liquids; Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; And why they adopt the shape. Solid Liquid Gas Compressibility.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Solid Liquid Gas Compressibility Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). Gases are compressible because most. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. The thermal expansion of liquids; Liquids can flow easily and assume the shape of their. Gases are highly compressible compared to. Solid Liquid Gas Compressibility.
From www.pinterest.com
States of Matter solids, liquids and gases Chemistry for All The Solid Liquid Gas Compressibility This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Solids maintain a fixed volume and shape and are not easily compressed. And why they adopt the shape (but not the volume) of their containers. Gases are compressible because most. Liquids can flow easily and assume the shape of their. Compressibility is the measure of. Solid Liquid Gas Compressibility.
From psiberg.com
Properties of Solid, Liquid, Gases A Comparison Solid Liquid Gas Compressibility The thermal expansion of liquids; Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Gases are compressible because most. Liquids can flow easily and assume the shape of their. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. Gases are very compressible, so. Solid Liquid Gas Compressibility.
From byjus.com
Which of the following shows Highest interpartical spaces Highest Solid Liquid Gas Compressibility Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). The thermal expansion of liquids; Gases have a low density because their particles are very far apart and occupy any given space, leading to a greater volume relative to the number of particles. Gases are compressible because most. This model explains the higher. Solid Liquid Gas Compressibility.
From www.slideserve.com
PPT CHAPTER 10 STATES OF MATTER PowerPoint Presentation, free Solid Liquid Gas Compressibility The thermal expansion of liquids; Gases are compressible because most. Liquids can flow easily and assume the shape of their. Solids maintain a fixed volume and shape and are not easily compressed. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. This model explains the higher density, greater order, and lower compressibility. Solid Liquid Gas Compressibility.
From www.yaclass.in
Compressibility of solids, liquids and gases — lesson. Science State Solid Liquid Gas Compressibility The thermal expansion of liquids; And why they adopt the shape (but not the volume) of their containers. Solids maintain a fixed volume and shape and are not easily compressed. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Heat, cool and compress atoms and molecules and watch as they change between solid, liquid. Solid Liquid Gas Compressibility.
From www.slideshare.net
Unit 1 Notes Solid Liquid Gas Compressibility Solids maintain a fixed volume and shape and are not easily compressed. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). Liquids can flow easily and assume the shape of their. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Heat, cool and compress atoms and. Solid Liquid Gas Compressibility.
From www.gasrebate.net
Properties Of Solids Liquids Gases Compared Teachoo Science Solid Liquid Gas Compressibility Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). Solids maintain a fixed volume and shape and are not easily compressed. Liquids can flow easily and assume the shape of their. Gases have a low density because their particles are very far apart and occupy any given space, leading to a greater. Solid Liquid Gas Compressibility.
From www.slideserve.com
PPT Matter PowerPoint Presentation, free download ID8890944 Solid Liquid Gas Compressibility This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Gases have a low density because their particles are very far apart and occupy any given space, leading to a greater volume relative to the number of particles.. Solid Liquid Gas Compressibility.
From nursehub.com
States of Matter NurseHub Solid Liquid Gas Compressibility Gases have a low density because their particles are very far apart and occupy any given space, leading to a greater volume relative to the number of particles. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Gases are highly compressible compared to solids and liquids due to the larger. Gases are. Solid Liquid Gas Compressibility.
From www.slideshare.net
States of matter Solid Liquid Gas Compressibility Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Gases are compressible because most. Gases are highly compressible compared to solids and liquids due to the larger. And why they adopt the shape (but not. Solid Liquid Gas Compressibility.
From middleschoolscience.com
Solid, Liquid, & Gas Triple Venn Diagram Activity Middle School Solid Liquid Gas Compressibility Liquids can flow easily and assume the shape of their. Gases have a low density because their particles are very far apart and occupy any given space, leading to a greater volume relative to the number of particles. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). Gases are highly compressible compared. Solid Liquid Gas Compressibility.
From www.numerade.com
SOLVEDExamine Figure 1.12 . In which physical state do particles have Solid Liquid Gas Compressibility Gases have a low density because their particles are very far apart and occupy any given space, leading to a greater volume relative to the number of particles. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Gases are highly compressible compared to solids and liquids due to the larger. Liquids can. Solid Liquid Gas Compressibility.
From www.slideshare.net
Compressibility of solids , liquids and gases Solid Liquid Gas Compressibility Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Gases are compressible because most. Gases have a low density because their particles are very far apart and occupy any given space, leading to a greater volume relative to the number of particles. And why they adopt the shape (but not the volume). Solid Liquid Gas Compressibility.
From www.slideserve.com
PPT Compressibility PowerPoint Presentation, free download ID1797453 Solid Liquid Gas Compressibility Gases have a low density because their particles are very far apart and occupy any given space, leading to a greater volume relative to the number of particles. And why they adopt the shape (but not the volume) of their containers. Gases are highly compressible compared to solids and liquids due to the larger. Heat, cool and compress atoms and. Solid Liquid Gas Compressibility.
From www.slideserve.com
PPT Chapter 11 Liquids & Solids PowerPoint Presentation, free Solid Liquid Gas Compressibility The thermal expansion of liquids; Solids maintain a fixed volume and shape and are not easily compressed. Liquids can flow easily and assume the shape of their. Gases are highly compressible compared to solids and liquids due to the larger. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. And why. Solid Liquid Gas Compressibility.
From igcsechemistryrevision.weebly.com
1.1 Understand the arrangement, movement and energy of particles in Solid Liquid Gas Compressibility Gases are highly compressible compared to solids and liquids due to the larger. Liquids can flow easily and assume the shape of their. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Compressibility is the measure. Solid Liquid Gas Compressibility.
From www.savemyexams.com
Solids, Liquids & Gases OCR A Level Physics Revision Notes 2017 Solid Liquid Gas Compressibility The thermal expansion of liquids; Solids maintain a fixed volume and shape and are not easily compressed. Gases are highly compressible compared to solids and liquids due to the larger. And why they adopt the shape (but not the volume) of their containers. Gases are compressible because most. Gases are very compressible, so when subjected to high pressures, their volumes. Solid Liquid Gas Compressibility.
From slideplayer.com
Properties of Gases. ppt download Solid Liquid Gas Compressibility Liquids can flow easily and assume the shape of their. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. And why they adopt the shape (but not the volume) of their containers. Gases are. Solid Liquid Gas Compressibility.
From www.animalia-life.club
Examples Of Solids Liquids And Gases Solid Liquid Gas Compressibility Solids maintain a fixed volume and shape and are not easily compressed. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Gases are highly compressible compared to solids and liquids due to the larger. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). And why they. Solid Liquid Gas Compressibility.
From slideplayer.com
Solids, Liquids, & Gases Chapter 7 ppt download Solid Liquid Gas Compressibility Liquids can flow easily and assume the shape of their. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Gases are highly compressible compared to solids and liquids due to the larger. Solids maintain a fixed volume and shape and are not easily compressed. This model explains the higher density, greater order,. Solid Liquid Gas Compressibility.
From blog.mensor.com
What Exactly is The Compressibility of Fluids? Solid Liquid Gas Compressibility Solids maintain a fixed volume and shape and are not easily compressed. Gases are highly compressible compared to solids and liquids due to the larger. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; The thermal expansion of liquids; Liquids can flow easily and assume the shape of their. And why they adopt the. Solid Liquid Gas Compressibility.
From dianaschemistrynotes.weebly.com
Chapter 2 CHEMISTRY NOTES Solid Liquid Gas Compressibility Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. The thermal expansion of liquids; And why they adopt the shape (but not the volume) of their containers. Gases are compressible because most. Solids maintain a fixed volume and shape and are not easily compressed. Gases are highly compressible compared to solids. Solid Liquid Gas Compressibility.
From socratic.org
What are examples of gases, liquids, and solids? Socratic Solid Liquid Gas Compressibility Gases are highly compressible compared to solids and liquids due to the larger. Gases are compressible because most. The thermal expansion of liquids; Gases have a low density because their particles are very far apart and occupy any given space, leading to a greater volume relative to the number of particles. Solids maintain a fixed volume and shape and are. Solid Liquid Gas Compressibility.
From www.yaclass.in
Compressibility of solids, liquids and gases — lesson. Science State Solid Liquid Gas Compressibility The thermal expansion of liquids; And why they adopt the shape (but not the volume) of their containers. Solids maintain a fixed volume and shape and are not easily compressed. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Gases are very compressible, so when subjected to high pressures, their volumes decrease. Solid Liquid Gas Compressibility.
From www.youtube.com
Compressibility of solid, liquid and gas YouTube Solid Liquid Gas Compressibility Solids maintain a fixed volume and shape and are not easily compressed. Gases are compressible because most. Gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Liquids can flow easily and assume the shape of their. Heat,. Solid Liquid Gas Compressibility.
From www.slideserve.com
PPT Matter & Energy PowerPoint Presentation, free download ID5368269 Solid Liquid Gas Compressibility Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. Liquids can flow easily and assume the shape of their. This model explains the higher density, greater order, and lower compressibility of liquids versus gases;. Solid Liquid Gas Compressibility.
From www.sciencefacts.net
Physics Page 7 of 19 Science Facts Solid Liquid Gas Compressibility Solids maintain a fixed volume and shape and are not easily compressed. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Gases are highly compressible compared to solids and liquids due to the larger. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. Gases are compressible. Solid Liquid Gas Compressibility.
From slideplayer.com
Unit 7 States of matter and the Behavior of Gases ppt download Solid Liquid Gas Compressibility Gases have a low density because their particles are very far apart and occupy any given space, leading to a greater volume relative to the number of particles. Solids maintain a fixed volume and shape and are not easily compressed. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. The thermal expansion. Solid Liquid Gas Compressibility.
From slideplayer.com
Liquids and Solids Gas Solid low density high compressibility ppt Solid Liquid Gas Compressibility Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. Compressibility is the measure of reducing the volume of a substance when pressure is applied to it. This model explains the higher density, greater order, and lower compressibility of liquids versus gases; Liquids can flow easily and assume the shape of their.. Solid Liquid Gas Compressibility.