Copper Electronic Arrangement . 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. understanding copper’s electron arrangement is crucial for exploring its chemical properties. one of the key factors that contributes to these properties is copper’s electron configuration. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. electronic configuration of cu. what is the electron configuration of copper. for example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. The electronic configuration of copper (cu) can be represented as:
from www.lifeder.com
understanding copper’s electron arrangement is crucial for exploring its chemical properties. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. electronic configuration of cu. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. for example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. what is the electron configuration of copper. one of the key factors that contributes to these properties is copper’s electron configuration. The electronic configuration of copper (cu) can be represented as: how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first.
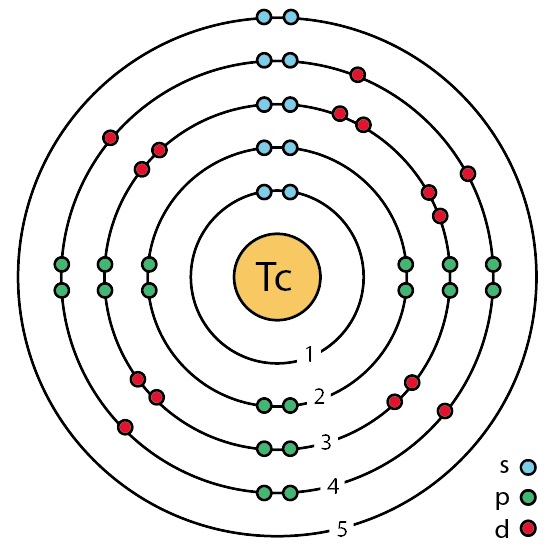
Tecnecio (Tc) estructura, propiedades, usos, obtención
Copper Electronic Arrangement 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. understanding copper’s electron arrangement is crucial for exploring its chemical properties. for example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. The electronic configuration of copper (cu) can be represented as: 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. electronic configuration of cu. one of the key factors that contributes to these properties is copper’s electron configuration. what is the electron configuration of copper. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.54C Explain Typical Physical Properties of Copper Electronic Arrangement Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. one of the key factors that contributes to these properties is copper’s electron configuration. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. what is the electron configuration of copper. electronic configuration of cu. for example, the electron configurations of the. Copper Electronic Arrangement.
From chem.libretexts.org
Introduction to Crystal Field Theory Chemistry LibreTexts Copper Electronic Arrangement what is the electron configuration of copper. one of the key factors that contributes to these properties is copper’s electron configuration. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. The electronic configuration of copper (cu) can be represented as: how to. Copper Electronic Arrangement.
From www.rapidmetals.co.uk
What are the Top Five Uses of Copper in the Industry Today? Rapid Metals Copper Electronic Arrangement one of the key factors that contributes to these properties is copper’s electron configuration. electronic configuration of cu. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. for example, the. Copper Electronic Arrangement.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Copper Electronic Arrangement understanding copper’s electron arrangement is crucial for exploring its chemical properties. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. The electronic configuration of copper (cu) can be represented as: how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. one. Copper Electronic Arrangement.
From chem.libretexts.org
Chapter 2.4 Electronic Structure of the Transition Metals Chemistry Copper Electronic Arrangement 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. for example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. The electronic configuration of copper (cu) can be represented as: what is the electron configuration of copper. electronic configuration of cu. Copper’s electron configuration. Copper Electronic Arrangement.
From ar.inspiredpencil.com
Copper Atomic Structure Copper Electronic Arrangement The electronic configuration of copper (cu) can be represented as: 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. electronic configuration of cu. for example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. what is the electron configuration of copper. one of. Copper Electronic Arrangement.
From www.slideserve.com
PPT 2.3 Electron Arrangement PowerPoint Presentation, free download Copper Electronic Arrangement for example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. what is the electron configuration of copper. one of the key factors that contributes to these properties is copper’s electron configuration. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. how to write the. Copper Electronic Arrangement.
From diagramlibraryschemer.z19.web.core.windows.net
Copper Electron Configuration Diagram Copper Electronic Arrangement Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. understanding copper’s electron arrangement is crucial for exploring its chemical properties. what is the electron configuration of copper. for example, the electron configurations. Copper Electronic Arrangement.
From schematicfixcinnamon.z5.web.core.windows.net
Electron Configuration Cu 1 Orbital Diagram Copper Electronic Arrangement Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. for example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. one of the key factors that contributes to these properties is copper’s electron configuration.. Copper Electronic Arrangement.
From baoge-machinery.en.made-in-china.com
Mild Steel Busbar Arrangement Panel, 300 AMP for High Conductive Copper Electronic Arrangement how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. electronic configuration of cu. one of the key factors that contributes to these properties is copper’s electron configuration. what is the electron configuration of copper. The electronic configuration of copper (cu) can be represented. Copper Electronic Arrangement.
From www.slideserve.com
PPT Electronic Configuration PowerPoint Presentation ID4748754 Copper Electronic Arrangement electronic configuration of cu. what is the electron configuration of copper. one of the key factors that contributes to these properties is copper’s electron configuration. for example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. how to write the electron configuration for copper (cu, cu+,. Copper Electronic Arrangement.
From www.sciencephoto.com
Copper, atomic structure Stock Image C046/0337 Science Photo Library Copper Electronic Arrangement The electronic configuration of copper (cu) can be represented as: what is the electron configuration of copper. understanding copper’s electron arrangement is crucial for exploring its chemical properties. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. electronic configuration of cu. . Copper Electronic Arrangement.
From www.toppr.com
The electronic configuration of copper (29Cu) is. Copper Electronic Arrangement Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. electronic configuration of cu. what is the electron configuration of copper. for example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. how. Copper Electronic Arrangement.
From www.youtube.com
Electron Configurations YouTube Copper Electronic Arrangement electronic configuration of cu. what is the electron configuration of copper. understanding copper’s electron arrangement is crucial for exploring its chemical properties. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. one of the key factors that contributes to these properties is. Copper Electronic Arrangement.
From schematicmaxeywheezle.z21.web.core.windows.net
Atomic Orbital Diagram For Copper Copper Electronic Arrangement understanding copper’s electron arrangement is crucial for exploring its chemical properties. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. for example, the electron configurations of the transition metals chromium (cr) and copper. Copper Electronic Arrangement.
From kaylumnegan.blogspot.com
36+ electronic configuration calculator KaylumNegan Copper Electronic Arrangement understanding copper’s electron arrangement is crucial for exploring its chemical properties. The electronic configuration of copper (cu) can be represented as: Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. what is the electron configuration of copper. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper. Copper Electronic Arrangement.
From spmchemistry.blog.onlinetuition.com.my
Formation of Ion SPM Chemistry Copper Electronic Arrangement Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. one of the key factors that contributes to these properties is copper’s electron configuration. understanding copper’s electron arrangement is crucial for exploring its chemical properties. electronic configuration of cu. The electronic configuration of copper (cu) can be represented as: what is the electron configuration of. Copper Electronic Arrangement.
From www.youtube.com
Copper Electron Configuration Organic Chemistry Examples YouTube Copper Electronic Arrangement how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. what is the electron configuration of copper. The electronic configuration of copper (cu) can be represented as: Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is. Copper Electronic Arrangement.
From periodictable.me
How To Find A Electron Configuration For Copper Dynamic Periodic Copper Electronic Arrangement electronic configuration of cu. one of the key factors that contributes to these properties is copper’s electron configuration. The electronic configuration of copper (cu) can be represented as: how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. for example, the electron configurations of. Copper Electronic Arrangement.
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Copper Electronic Arrangement what is the electron configuration of copper. one of the key factors that contributes to these properties is copper’s electron configuration. The electronic configuration of copper (cu) can be represented as: Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to. Copper Electronic Arrangement.
From dxojzvsyh.blob.core.windows.net
Copper Electron Cloud Model at Wallace Miles blog Copper Electronic Arrangement The electronic configuration of copper (cu) can be represented as: Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. electronic configuration of cu. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. understanding copper’s electron arrangement is crucial for exploring its chemical properties. for example, the electron configurations of the transition. Copper Electronic Arrangement.
From www.bharatagritech.com
Electronic Configuration Of Copper Discounted Deals www Copper Electronic Arrangement how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. what is the electron configuration of copper. The electronic configuration of copper (cu) can be represented as: understanding copper’s electron arrangement is. Copper Electronic Arrangement.
From myans.bhantedhammika.net
Electron Configuration Worksheet Answer Key Copper Copper Electronic Arrangement 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. for example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. electronic configuration of cu. what is the electron configuration of copper. The electronic configuration of copper (cu) can be represented as: how to. Copper Electronic Arrangement.
From www.schoolmykids.com
Iron (Fe) Element Information, Facts, Properties, Uses Periodic Copper Electronic Arrangement what is the electron configuration of copper. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. for example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. understanding copper’s electron arrangement is crucial for exploring its chemical properties. one of the key factors that contributes. Copper Electronic Arrangement.
From chem2u.blogspot.my
chem2U November 2013 Copper Electronic Arrangement one of the key factors that contributes to these properties is copper’s electron configuration. The electronic configuration of copper (cu) can be represented as: understanding copper’s electron arrangement is crucial for exploring its chemical properties. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first.. Copper Electronic Arrangement.
From lessonschoolintimistes.z14.web.core.windows.net
W 3+ Electron Configuration Copper Electronic Arrangement 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. The electronic configuration of copper (cu) can be represented as: electronic configuration. Copper Electronic Arrangement.
From www.alamy.com
Symbol and electron diagram for Copper Stock Vector Image & Art Alamy Copper Electronic Arrangement for example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. electronic configuration of cu. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. The electronic configuration of copper (cu) can be represented as: one of the key factors that contributes to these properties. Copper Electronic Arrangement.
From valenceelectrons.com
Copper(Cu) electron configuration and orbital diagram Copper Electronic Arrangement Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. for example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. what is the electron configuration of copper. The electronic configuration of copper (cu) can be represented as: how to write the electron configuration for copper (cu,. Copper Electronic Arrangement.
From valenceelectrons.com
How to Find the Valence Electrons for Titanium (Ti)? Copper Electronic Arrangement what is the electron configuration of copper. electronic configuration of cu. The electronic configuration of copper (cu) can be represented as: understanding copper’s electron arrangement is crucial for exploring its chemical properties. for example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. how to write. Copper Electronic Arrangement.
From chem2u.blogspot.com
chem2U Arrangement of Atoms in Bronze Copper Electronic Arrangement what is the electron configuration of copper. electronic configuration of cu. one of the key factors that contributes to these properties is copper’s electron configuration. The electronic configuration of copper (cu) can be represented as: for example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. . Copper Electronic Arrangement.
From www.slideshare.net
Copper Copper Electronic Arrangement Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. electronic configuration of cu. for example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. The electronic configuration of copper (cu) can be represented as:. Copper Electronic Arrangement.
From wiringguidefrosts.z19.web.core.windows.net
Copper Electron Configuration Diagram Copper Electronic Arrangement one of the key factors that contributes to these properties is copper’s electron configuration. electronic configuration of cu. for example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. what is the electron configuration of copper. how to write the electron configuration for copper (cu, cu+,. Copper Electronic Arrangement.
From kethewtdudley.blogspot.com
Electronic Configuration of Copper KethewtDudley Copper Electronic Arrangement one of the key factors that contributes to these properties is copper’s electron configuration. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. The electronic configuration of copper (cu) can be represented as: Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. for example, the electron configurations of the transition metals chromium. Copper Electronic Arrangement.
From www.lifeder.com
Tecnecio (Tc) estructura, propiedades, usos, obtención Copper Electronic Arrangement how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. for example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. electronic configuration of cu.. Copper Electronic Arrangement.
From www.bharatagritech.com
Electronic Configuration Of Copper Discounted Deals www Copper Electronic Arrangement for example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. one of the key factors that contributes to these properties is copper’s electron configuration. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling. The electronic configuration of copper (cu) can be represented as: what is. Copper Electronic Arrangement.