Does Increasing Temperature Increase Vapor Pressure . As the temperature of a liquid or solid increases its vapor pressure also increases. At a pressure greater than 1 atm, water boils at a temperature greater than 100°c because the increased pressure forces vapor. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. As temperature increases the molecular activity at the surface of the water would increase. According to the kinetic molecular theory, the temperature reflects the average kinetic energy of the particles in a. The natural logarithm (ln) changes the nonlinear relationship between. Vapour pressure is defined as the pressure exerted by the gas molecules on the surface of the liquid it is in equilibrium with at a.
from www.alamy.com
Vapour pressure is defined as the pressure exerted by the gas molecules on the surface of the liquid it is in equilibrium with at a. As temperature increases the molecular activity at the surface of the water would increase. According to the kinetic molecular theory, the temperature reflects the average kinetic energy of the particles in a. At a pressure greater than 1 atm, water boils at a temperature greater than 100°c because the increased pressure forces vapor. As the temperature of a liquid or solid increases its vapor pressure also increases. The natural logarithm (ln) changes the nonlinear relationship between. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e.
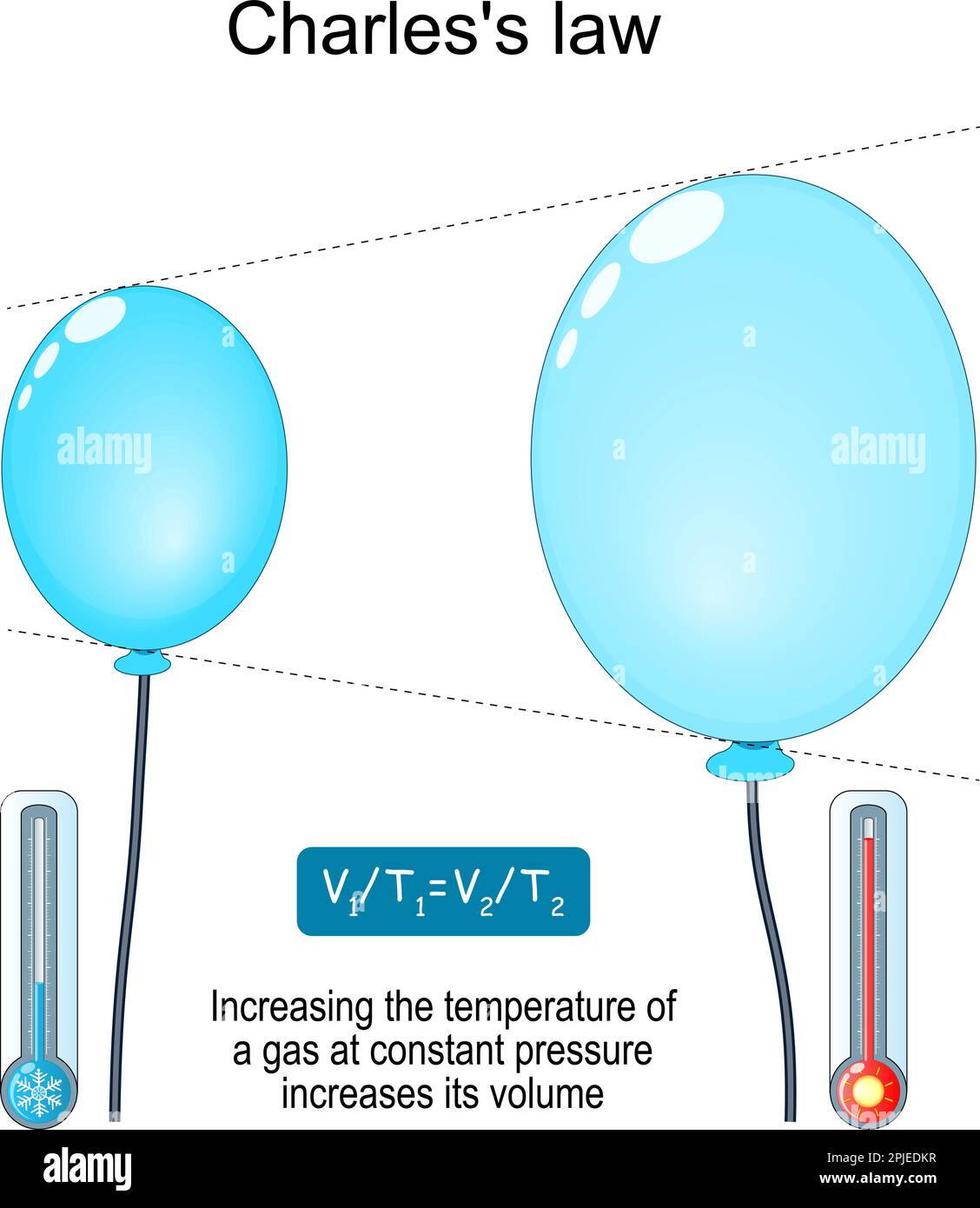
Charles's law. relationship between volume and temperature. Increasing the temperature of a gas
Does Increasing Temperature Increase Vapor Pressure Vapour pressure is defined as the pressure exerted by the gas molecules on the surface of the liquid it is in equilibrium with at a. As temperature increases the molecular activity at the surface of the water would increase. Vapour pressure is defined as the pressure exerted by the gas molecules on the surface of the liquid it is in equilibrium with at a. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The natural logarithm (ln) changes the nonlinear relationship between. As the temperature of a liquid or solid increases its vapor pressure also increases. According to the kinetic molecular theory, the temperature reflects the average kinetic energy of the particles in a. At a pressure greater than 1 atm, water boils at a temperature greater than 100°c because the increased pressure forces vapor.
From www.quora.com
If temperature increases, pressure increases. Does temperature increase if pressure increases Does Increasing Temperature Increase Vapor Pressure Vapour pressure is defined as the pressure exerted by the gas molecules on the surface of the liquid it is in equilibrium with at a. As temperature increases the molecular activity at the surface of the water would increase. According to the kinetic molecular theory, the temperature reflects the average kinetic energy of the particles in a. As the temperature. Does Increasing Temperature Increase Vapor Pressure.
From slideplayer.com
Vapor Pressure Vaporization change from liquid to gas at boiling point. Evaporation change Does Increasing Temperature Increase Vapor Pressure Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. At a pressure greater than 1 atm, water boils at a temperature greater than 100°c because the increased pressure forces vapor. The natural logarithm (ln) changes the nonlinear relationship between. As temperature increases the molecular activity at the surface of the water would increase. As. Does Increasing Temperature Increase Vapor Pressure.
From techblog.ctgclean.com
Drying Vapor Pressure vs. Temperature CTG Clean Does Increasing Temperature Increase Vapor Pressure Vapour pressure is defined as the pressure exerted by the gas molecules on the surface of the liquid it is in equilibrium with at a. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. As temperature increases the molecular activity at the surface of the water would increase. As the temperature of a liquid. Does Increasing Temperature Increase Vapor Pressure.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It Does Increasing Temperature Increase Vapor Pressure According to the kinetic molecular theory, the temperature reflects the average kinetic energy of the particles in a. At a pressure greater than 1 atm, water boils at a temperature greater than 100°c because the increased pressure forces vapor. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The natural logarithm (ln) changes the. Does Increasing Temperature Increase Vapor Pressure.
From slideplayer.com
Aim How does temperature affect the vapor pressure of liquids? Essential Questions Why does Does Increasing Temperature Increase Vapor Pressure Vapour pressure is defined as the pressure exerted by the gas molecules on the surface of the liquid it is in equilibrium with at a. As temperature increases the molecular activity at the surface of the water would increase. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. As the temperature of a liquid. Does Increasing Temperature Increase Vapor Pressure.
From mungfali.com
Water Vapor Pressure Temperature Chart Does Increasing Temperature Increase Vapor Pressure As the temperature of a liquid or solid increases its vapor pressure also increases. According to the kinetic molecular theory, the temperature reflects the average kinetic energy of the particles in a. As temperature increases the molecular activity at the surface of the water would increase. At a pressure greater than 1 atm, water boils at a temperature greater than. Does Increasing Temperature Increase Vapor Pressure.
From www.slideserve.com
PPT Why . . . PowerPoint Presentation, free download ID2250075 Does Increasing Temperature Increase Vapor Pressure As the temperature of a liquid or solid increases its vapor pressure also increases. The natural logarithm (ln) changes the nonlinear relationship between. Vapour pressure is defined as the pressure exerted by the gas molecules on the surface of the liquid it is in equilibrium with at a. According to the kinetic molecular theory, the temperature reflects the average kinetic. Does Increasing Temperature Increase Vapor Pressure.
From www.slideserve.com
PPT Vapor and it Pressure PowerPoint Presentation, free download ID9097394 Does Increasing Temperature Increase Vapor Pressure The natural logarithm (ln) changes the nonlinear relationship between. As temperature increases the molecular activity at the surface of the water would increase. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. As the temperature of a liquid or solid increases its vapor pressure also increases. Vapour pressure is defined as the pressure exerted. Does Increasing Temperature Increase Vapor Pressure.
From www.slideserve.com
PPT Chapter 11 Liquids & Solids PowerPoint Presentation, free download ID1623857 Does Increasing Temperature Increase Vapor Pressure According to the kinetic molecular theory, the temperature reflects the average kinetic energy of the particles in a. As the temperature of a liquid or solid increases its vapor pressure also increases. Vapour pressure is defined as the pressure exerted by the gas molecules on the surface of the liquid it is in equilibrium with at a. As temperature increases. Does Increasing Temperature Increase Vapor Pressure.
From www.slideserve.com
PPT 17 Equilibrium (AHL) PowerPoint Presentation, free download ID2464656 Does Increasing Temperature Increase Vapor Pressure As temperature increases the molecular activity at the surface of the water would increase. At a pressure greater than 1 atm, water boils at a temperature greater than 100°c because the increased pressure forces vapor. The natural logarithm (ln) changes the nonlinear relationship between. According to the kinetic molecular theory, the temperature reflects the average kinetic energy of the particles. Does Increasing Temperature Increase Vapor Pressure.
From shaunmwilliams.com
Chapter 10 Presentation Does Increasing Temperature Increase Vapor Pressure Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. As the temperature of a liquid or solid increases its vapor pressure also increases. Vapour pressure is defined as the pressure exerted by the gas molecules on the surface of the liquid it is in equilibrium with at a. As temperature increases the molecular activity. Does Increasing Temperature Increase Vapor Pressure.
From socratic.org
Explain how each of the following affects the vapour pressure of a liquid (a) volume of the Does Increasing Temperature Increase Vapor Pressure According to the kinetic molecular theory, the temperature reflects the average kinetic energy of the particles in a. As the temperature of a liquid or solid increases its vapor pressure also increases. Vapour pressure is defined as the pressure exerted by the gas molecules on the surface of the liquid it is in equilibrium with at a. The natural logarithm. Does Increasing Temperature Increase Vapor Pressure.
From slideplayer.com
Aim How does temperature affect the vapor pressure of liquids? Essential Questions Why does Does Increasing Temperature Increase Vapor Pressure As temperature increases the molecular activity at the surface of the water would increase. According to the kinetic molecular theory, the temperature reflects the average kinetic energy of the particles in a. The natural logarithm (ln) changes the nonlinear relationship between. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. As the temperature of. Does Increasing Temperature Increase Vapor Pressure.
From pressbooks.bccampus.ca
Unit 4 The Respiratory System Douglas College Human Anatomy & Physiology II (2nd ed.) Does Increasing Temperature Increase Vapor Pressure At a pressure greater than 1 atm, water boils at a temperature greater than 100°c because the increased pressure forces vapor. The natural logarithm (ln) changes the nonlinear relationship between. As temperature increases the molecular activity at the surface of the water would increase. As the temperature of a liquid or solid increases its vapor pressure also increases. According to. Does Increasing Temperature Increase Vapor Pressure.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 Does Increasing Temperature Increase Vapor Pressure According to the kinetic molecular theory, the temperature reflects the average kinetic energy of the particles in a. As the temperature of a liquid or solid increases its vapor pressure also increases. The natural logarithm (ln) changes the nonlinear relationship between. At a pressure greater than 1 atm, water boils at a temperature greater than 100°c because the increased pressure. Does Increasing Temperature Increase Vapor Pressure.
From slideplayer.com
Aim How does temperature affect the vapor pressure of liquids? Essential Questions Why does Does Increasing Temperature Increase Vapor Pressure The natural logarithm (ln) changes the nonlinear relationship between. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. As temperature increases the molecular activity at the surface of the water would increase. Vapour pressure is defined as the pressure exerted by the gas molecules on the surface of the liquid it is in equilibrium. Does Increasing Temperature Increase Vapor Pressure.
From www.slideserve.com
PPT Vapor and it Pressure PowerPoint Presentation, free download ID9097394 Does Increasing Temperature Increase Vapor Pressure At a pressure greater than 1 atm, water boils at a temperature greater than 100°c because the increased pressure forces vapor. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. As the temperature of a liquid or solid increases its vapor pressure also increases. Vapour pressure is defined as the pressure exerted by the. Does Increasing Temperature Increase Vapor Pressure.
From www.gauthmath.com
Solved What is the relationship of vapor pressure to temperature of a liquid? a As temperature Does Increasing Temperature Increase Vapor Pressure Vapour pressure is defined as the pressure exerted by the gas molecules on the surface of the liquid it is in equilibrium with at a. As temperature increases the molecular activity at the surface of the water would increase. As the temperature of a liquid or solid increases its vapor pressure also increases. The natural logarithm (ln) changes the nonlinear. Does Increasing Temperature Increase Vapor Pressure.
From nigerianscholars.com
Phase Changes Temperature, Theory, and Gas Laws Does Increasing Temperature Increase Vapor Pressure The natural logarithm (ln) changes the nonlinear relationship between. At a pressure greater than 1 atm, water boils at a temperature greater than 100°c because the increased pressure forces vapor. As temperature increases the molecular activity at the surface of the water would increase. According to the kinetic molecular theory, the temperature reflects the average kinetic energy of the particles. Does Increasing Temperature Increase Vapor Pressure.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download ID6717792 Does Increasing Temperature Increase Vapor Pressure As temperature increases the molecular activity at the surface of the water would increase. Vapour pressure is defined as the pressure exerted by the gas molecules on the surface of the liquid it is in equilibrium with at a. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. As the temperature of a liquid. Does Increasing Temperature Increase Vapor Pressure.
From jmpcoblog.com
Avoiding Pump Cavitation in Open Systems The Dynamic Relationship Between NPSH, Vapor Pressure Does Increasing Temperature Increase Vapor Pressure At a pressure greater than 1 atm, water boils at a temperature greater than 100°c because the increased pressure forces vapor. According to the kinetic molecular theory, the temperature reflects the average kinetic energy of the particles in a. Vapour pressure is defined as the pressure exerted by the gas molecules on the surface of the liquid it is in. Does Increasing Temperature Increase Vapor Pressure.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Does Increasing Temperature Increase Vapor Pressure As temperature increases the molecular activity at the surface of the water would increase. The natural logarithm (ln) changes the nonlinear relationship between. At a pressure greater than 1 atm, water boils at a temperature greater than 100°c because the increased pressure forces vapor. Vapour pressure is defined as the pressure exerted by the gas molecules on the surface of. Does Increasing Temperature Increase Vapor Pressure.
From www.teachoo.com
Changing Pressure to Change State of Matter Chemistry Teachoo Does Increasing Temperature Increase Vapor Pressure According to the kinetic molecular theory, the temperature reflects the average kinetic energy of the particles in a. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. As temperature increases the molecular activity at the surface of the water would increase. Vapour pressure is defined as the pressure exerted by the gas molecules on. Does Increasing Temperature Increase Vapor Pressure.
From www.slideserve.com
PPT Unit 4 Phases of Matter (Chapters 1314) PowerPoint Presentation ID3326407 Does Increasing Temperature Increase Vapor Pressure Vapour pressure is defined as the pressure exerted by the gas molecules on the surface of the liquid it is in equilibrium with at a. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. At a pressure greater than 1 atm, water boils at a temperature greater than 100°c because the increased pressure forces. Does Increasing Temperature Increase Vapor Pressure.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Does Increasing Temperature Increase Vapor Pressure As temperature increases the molecular activity at the surface of the water would increase. Vapour pressure is defined as the pressure exerted by the gas molecules on the surface of the liquid it is in equilibrium with at a. The natural logarithm (ln) changes the nonlinear relationship between. According to the kinetic molecular theory, the temperature reflects the average kinetic. Does Increasing Temperature Increase Vapor Pressure.
From www.savemyexams.co.uk
Gas Law Relationships (1.2.5) IB DP Chemistry SL Revision Notes 2016 Save My Exams Does Increasing Temperature Increase Vapor Pressure As the temperature of a liquid or solid increases its vapor pressure also increases. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. At a pressure greater than 1 atm, water boils at a temperature greater than 100°c because the increased pressure forces vapor. As temperature increases the molecular activity at the surface of. Does Increasing Temperature Increase Vapor Pressure.
From www.slideserve.com
PPT Vapor and it Pressure PowerPoint Presentation, free download ID9097394 Does Increasing Temperature Increase Vapor Pressure The natural logarithm (ln) changes the nonlinear relationship between. According to the kinetic molecular theory, the temperature reflects the average kinetic energy of the particles in a. As the temperature of a liquid or solid increases its vapor pressure also increases. Vapour pressure is defined as the pressure exerted by the gas molecules on the surface of the liquid it. Does Increasing Temperature Increase Vapor Pressure.
From www.alamy.com
Charles's law. relationship between volume and temperature. Increasing the temperature of a gas Does Increasing Temperature Increase Vapor Pressure As temperature increases the molecular activity at the surface of the water would increase. The natural logarithm (ln) changes the nonlinear relationship between. At a pressure greater than 1 atm, water boils at a temperature greater than 100°c because the increased pressure forces vapor. According to the kinetic molecular theory, the temperature reflects the average kinetic energy of the particles. Does Increasing Temperature Increase Vapor Pressure.
From www.slideserve.com
PPT Unit 3 Phase Changes & Energy PowerPoint Presentation, free download ID3721071 Does Increasing Temperature Increase Vapor Pressure As the temperature of a liquid or solid increases its vapor pressure also increases. According to the kinetic molecular theory, the temperature reflects the average kinetic energy of the particles in a. At a pressure greater than 1 atm, water boils at a temperature greater than 100°c because the increased pressure forces vapor. Vapour pressure is defined as the pressure. Does Increasing Temperature Increase Vapor Pressure.
From www.slideserve.com
PPT States of Matter and Intermolecular Forces PowerPoint Presentation ID9138007 Does Increasing Temperature Increase Vapor Pressure The natural logarithm (ln) changes the nonlinear relationship between. Vapour pressure is defined as the pressure exerted by the gas molecules on the surface of the liquid it is in equilibrium with at a. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. At a pressure greater than 1 atm, water boils at a. Does Increasing Temperature Increase Vapor Pressure.
From www.researchgate.net
The effect of temperature on saturation vapor pressure, actual vapor... Download Scientific Does Increasing Temperature Increase Vapor Pressure The natural logarithm (ln) changes the nonlinear relationship between. As temperature increases the molecular activity at the surface of the water would increase. At a pressure greater than 1 atm, water boils at a temperature greater than 100°c because the increased pressure forces vapor. As the temperature of a liquid or solid increases its vapor pressure also increases. According to. Does Increasing Temperature Increase Vapor Pressure.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation ID705859 Does Increasing Temperature Increase Vapor Pressure Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. As temperature increases the molecular activity at the surface of the water would increase. Vapour pressure is defined as the pressure exerted by the gas molecules on the surface of the liquid it is in equilibrium with at a. The natural logarithm (ln) changes the. Does Increasing Temperature Increase Vapor Pressure.
From slideplayer.com
WarmUp. ppt download Does Increasing Temperature Increase Vapor Pressure At a pressure greater than 1 atm, water boils at a temperature greater than 100°c because the increased pressure forces vapor. As temperature increases the molecular activity at the surface of the water would increase. As the temperature of a liquid or solid increases its vapor pressure also increases. Vapour pressure is defined as the pressure exerted by the gas. Does Increasing Temperature Increase Vapor Pressure.
From www.slideserve.com
PPT Chapter 18 PowerPoint Presentation, free download ID1757364 Does Increasing Temperature Increase Vapor Pressure As temperature increases the molecular activity at the surface of the water would increase. As the temperature of a liquid or solid increases its vapor pressure also increases. At a pressure greater than 1 atm, water boils at a temperature greater than 100°c because the increased pressure forces vapor. The natural logarithm (ln) changes the nonlinear relationship between. According to. Does Increasing Temperature Increase Vapor Pressure.
From slideplayer.com
Aim How do gas molecules react to pressure, volume and temperature? ppt download Does Increasing Temperature Increase Vapor Pressure As temperature increases the molecular activity at the surface of the water would increase. Generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. At a pressure greater than 1 atm, water boils at a temperature greater than 100°c because the increased pressure forces vapor. Vapour pressure is defined as the pressure exerted by the. Does Increasing Temperature Increase Vapor Pressure.