How Does Salt Dissociate In Water . The positive areas of the water molecules surround the negative chloride ions. The negative areas of the water molecules surround the. The science of salt dissolving in water • salt water chemistry • learn how salt dissolves in water through the process of ionization,. Materials such as sodium chloride or calcium chloride are frequently employed for this. When salt is added to water, the positive and negative ions separate. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. We finally know in detail how salt dissolves in water. The process of dissolving involves the breaking up of a solute (salt) into individual particles and dispersing them throughout a. The water molecules surround the ions and pull them. Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then. One common approach to melting the ice is to put some form of deicing salt on the surface.
from masterconceptsinchemistry.com
The positive areas of the water molecules surround the negative chloride ions. When salt is added to water, the positive and negative ions separate. The science of salt dissolving in water • salt water chemistry • learn how salt dissolves in water through the process of ionization,. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then. One common approach to melting the ice is to put some form of deicing salt on the surface. The negative areas of the water molecules surround the. We finally know in detail how salt dissolves in water. The water molecules surround the ions and pull them. Materials such as sodium chloride or calcium chloride are frequently employed for this.
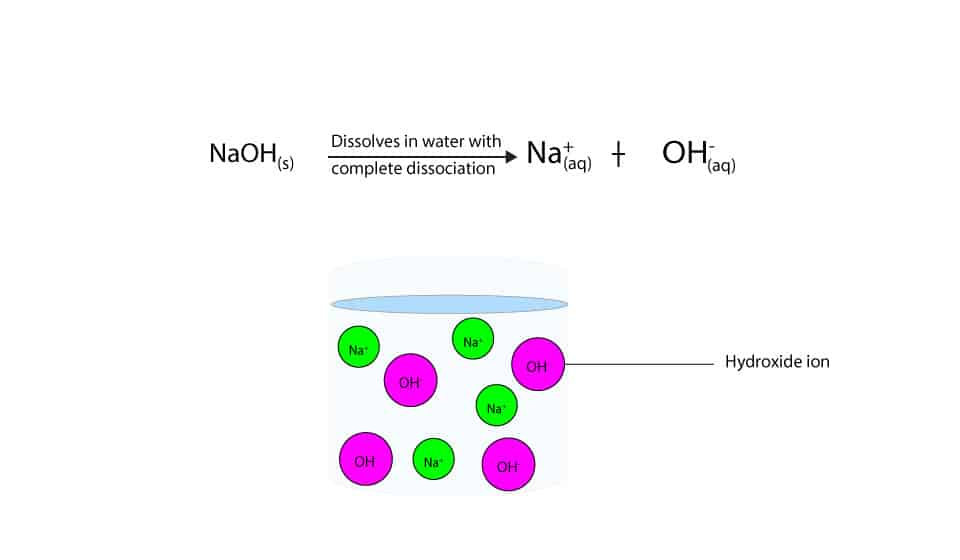
Why’s sodium hydroxide a strong base, while ammonia a weak base?
How Does Salt Dissociate In Water The water molecules surround the ions and pull them. The positive areas of the water molecules surround the negative chloride ions. Materials such as sodium chloride or calcium chloride are frequently employed for this. The science of salt dissolving in water • salt water chemistry • learn how salt dissolves in water through the process of ionization,. Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then. One common approach to melting the ice is to put some form of deicing salt on the surface. The negative areas of the water molecules surround the. The process of dissolving involves the breaking up of a solute (salt) into individual particles and dispersing them throughout a. The water molecules surround the ions and pull them. When salt is added to water, the positive and negative ions separate. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. We finally know in detail how salt dissolves in water.
From www.youtube.com
How does salt dissolve in water? YouTube How Does Salt Dissociate In Water The negative areas of the water molecules surround the. When salt is added to water, the positive and negative ions separate. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. One common approach to melting the ice is to put some form of deicing salt on the surface. Materials. How Does Salt Dissociate In Water.
From sciencenotes.org
Dissociation (Chemistry) Definition and Examples How Does Salt Dissociate In Water The process of dissolving involves the breaking up of a solute (salt) into individual particles and dispersing them throughout a. The positive areas of the water molecules surround the negative chloride ions. The water molecules surround the ions and pull them. Materials such as sodium chloride or calcium chloride are frequently employed for this. The science of salt dissolving in. How Does Salt Dissociate In Water.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download How Does Salt Dissociate In Water The water molecules surround the ions and pull them. When salt is added to water, the positive and negative ions separate. We finally know in detail how salt dissolves in water. The science of salt dissolving in water • salt water chemistry • learn how salt dissolves in water through the process of ionization,. The positive areas of the water. How Does Salt Dissociate In Water.
From www.youtube.com
How Salt Dissolves in Water? YouTube How Does Salt Dissociate In Water When salt is added to water, the positive and negative ions separate. Materials such as sodium chloride or calcium chloride are frequently employed for this. The positive areas of the water molecules surround the negative chloride ions. The science of salt dissolving in water • salt water chemistry • learn how salt dissolves in water through the process of ionization,.. How Does Salt Dissociate In Water.
From www.shalom-education.com
Acids and Alkalis GCSE Chemistry Revision How Does Salt Dissociate In Water We finally know in detail how salt dissolves in water. The science of salt dissolving in water • salt water chemistry • learn how salt dissolves in water through the process of ionization,. The positive areas of the water molecules surround the negative chloride ions. One common approach to melting the ice is to put some form of deicing salt. How Does Salt Dissociate In Water.
From www.slideserve.com
PPT The Nature of Aqueous Solutions and Molarity and Solution How Does Salt Dissociate In Water We finally know in detail how salt dissolves in water. The negative areas of the water molecules surround the. The process of dissolving involves the breaking up of a solute (salt) into individual particles and dispersing them throughout a. One common approach to melting the ice is to put some form of deicing salt on the surface. A machine learning. How Does Salt Dissociate In Water.
From www.slideserve.com
PPT Dissociation of water PowerPoint Presentation, free download ID How Does Salt Dissociate In Water One common approach to melting the ice is to put some form of deicing salt on the surface. The process of dissolving involves the breaking up of a solute (salt) into individual particles and dispersing them throughout a. Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then. Materials such as sodium chloride or calcium. How Does Salt Dissociate In Water.
From www.slideserve.com
PPT Solubility Rules & Net Ionic Equations PowerPoint Presentation How Does Salt Dissociate In Water The negative areas of the water molecules surround the. We finally know in detail how salt dissolves in water. When salt is added to water, the positive and negative ions separate. Materials such as sodium chloride or calcium chloride are frequently employed for this. The process of dissolving involves the breaking up of a solute (salt) into individual particles and. How Does Salt Dissociate In Water.
From masterconceptsinchemistry.com
Why’s sodium hydroxide a strong base, while ammonia a weak base? How Does Salt Dissociate In Water Materials such as sodium chloride or calcium chloride are frequently employed for this. When salt is added to water, the positive and negative ions separate. The positive areas of the water molecules surround the negative chloride ions. One common approach to melting the ice is to put some form of deicing salt on the surface. The water molecules surround the. How Does Salt Dissociate In Water.
From www.youtube.com
Dissolving Salt in Water YouTube How Does Salt Dissociate In Water We finally know in detail how salt dissolves in water. The positive areas of the water molecules surround the negative chloride ions. One common approach to melting the ice is to put some form of deicing salt on the surface. The negative areas of the water molecules surround the. When salt is added to water, the positive and negative ions. How Does Salt Dissociate In Water.
From byjus.com
Acid Dissociation An Overview of Acid Dissociation and Ka with FAQs How Does Salt Dissociate In Water Materials such as sodium chloride or calcium chloride are frequently employed for this. We finally know in detail how salt dissolves in water. The positive areas of the water molecules surround the negative chloride ions. One common approach to melting the ice is to put some form of deicing salt on the surface. The process of dissolving involves the breaking. How Does Salt Dissociate In Water.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free How Does Salt Dissociate In Water The science of salt dissolving in water • salt water chemistry • learn how salt dissolves in water through the process of ionization,. The negative areas of the water molecules surround the. The positive areas of the water molecules surround the negative chloride ions. We finally know in detail how salt dissolves in water. The process of dissolving involves the. How Does Salt Dissociate In Water.
From www.youtube.com
How does water dissolve salt? YouTube How Does Salt Dissociate In Water The negative areas of the water molecules surround the. When salt is added to water, the positive and negative ions separate. We finally know in detail how salt dissolves in water. The water molecules surround the ions and pull them. Materials such as sodium chloride or calcium chloride are frequently employed for this. The process of dissolving involves the breaking. How Does Salt Dissociate In Water.
From www.slideserve.com
PPT Physical Science PowerPoint Presentation, free download ID794118 How Does Salt Dissociate In Water The negative areas of the water molecules surround the. Materials such as sodium chloride or calcium chloride are frequently employed for this. The process of dissolving involves the breaking up of a solute (salt) into individual particles and dispersing them throughout a. When salt is added to water, the positive and negative ions separate. The water molecules surround the ions. How Does Salt Dissociate In Water.
From www.slideserve.com
PPT SPONCH PowerPoint Presentation, free download ID556676 How Does Salt Dissociate In Water One common approach to melting the ice is to put some form of deicing salt on the surface. The positive areas of the water molecules surround the negative chloride ions. Materials such as sodium chloride or calcium chloride are frequently employed for this. The water molecules surround the ions and pull them. A machine learning model has revealed how crystals. How Does Salt Dissociate In Water.
From sphweb.bumc.bu.edu
Chemical Elements Atoms How Does Salt Dissociate In Water When salt is added to water, the positive and negative ions separate. The water molecules surround the ions and pull them. The science of salt dissolving in water • salt water chemistry • learn how salt dissolves in water through the process of ionization,. One common approach to melting the ice is to put some form of deicing salt on. How Does Salt Dissociate In Water.
From masterconceptsinchemistry.com
How does sodium chloride (NaCl) dissolve in water? How Does Salt Dissociate In Water A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. The process of dissolving involves the breaking up of a solute (salt) into individual particles and dispersing them throughout a. The water molecules surround the ions and pull them. Water molecules pulling apart the ions (sodium and chloride) in a. How Does Salt Dissociate In Water.
From www.vectorstock.com
How does sodium chloride nacl dissolve in water Vector Image How Does Salt Dissociate In Water Materials such as sodium chloride or calcium chloride are frequently employed for this. Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then. The negative areas of the water molecules surround the. We finally know in detail how salt dissolves in water. The positive areas of the water molecules surround the negative chloride ions. The. How Does Salt Dissociate In Water.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID How Does Salt Dissociate In Water The science of salt dissolving in water • salt water chemistry • learn how salt dissolves in water through the process of ionization,. One common approach to melting the ice is to put some form of deicing salt on the surface. When salt is added to water, the positive and negative ions separate. Water molecules pulling apart the ions (sodium. How Does Salt Dissociate In Water.
From www.slideserve.com
PPT Na + PowerPoint Presentation, free download ID3020290 How Does Salt Dissociate In Water Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then. When salt is added to water, the positive and negative ions separate. The water molecules surround the ions and pull them. One common approach to melting the ice is to put some form of deicing salt on the surface. A machine learning model has revealed. How Does Salt Dissociate In Water.
From www.slideserve.com
PPT BASIC’S OF SOLUTION CHEMISTRY PowerPoint Presentation, free How Does Salt Dissociate In Water When salt is added to water, the positive and negative ions separate. The process of dissolving involves the breaking up of a solute (salt) into individual particles and dispersing them throughout a. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. We finally know in detail how salt dissolves. How Does Salt Dissociate In Water.
From schematicfixtrysted.z22.web.core.windows.net
Diagram Of Salt Dissolving In Water How Does Salt Dissociate In Water Materials such as sodium chloride or calcium chloride are frequently employed for this. When salt is added to water, the positive and negative ions separate. The process of dissolving involves the breaking up of a solute (salt) into individual particles and dispersing them throughout a. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then. How Does Salt Dissociate In Water.
From courses.lumenlearning.com
Water BIO103 Human Biology How Does Salt Dissociate In Water A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. We finally know in detail how salt dissolves in water. When salt is added to water, the positive and negative ions separate. The water molecules surround the ions and pull them. One common approach to melting the ice is to. How Does Salt Dissociate In Water.
From www.nagwa.com
Question Video Identifying the Salt That Would Produce a Basic How Does Salt Dissociate In Water The negative areas of the water molecules surround the. The positive areas of the water molecules surround the negative chloride ions. The science of salt dissolving in water • salt water chemistry • learn how salt dissolves in water through the process of ionization,. Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then. The. How Does Salt Dissociate In Water.
From www.slideserve.com
PPT Chapter 2 Chemistry PowerPoint Presentation, free download ID How Does Salt Dissociate In Water We finally know in detail how salt dissolves in water. The negative areas of the water molecules surround the. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. One common approach to melting the ice is to put some form of deicing salt on the surface. Materials such as. How Does Salt Dissociate In Water.
From www.youtube.com
How to (dissolving salt in water) YouTube How Does Salt Dissociate In Water A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. The positive areas of the water molecules surround the negative chloride ions. Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then. When salt is added to water, the positive and negative ions separate. The. How Does Salt Dissociate In Water.
From www.hamiltoncompany.com
Ions and Dissociation Process Analytics How Does Salt Dissociate In Water One common approach to melting the ice is to put some form of deicing salt on the surface. The science of salt dissolving in water • salt water chemistry • learn how salt dissolves in water through the process of ionization,. The positive areas of the water molecules surround the negative chloride ions. The water molecules surround the ions and. How Does Salt Dissociate In Water.
From www.expii.com
Good Solvent (Water) — Properties & Examples Expii How Does Salt Dissociate In Water The positive areas of the water molecules surround the negative chloride ions. We finally know in detail how salt dissolves in water. The negative areas of the water molecules surround the. One common approach to melting the ice is to put some form of deicing salt on the surface. A machine learning model has revealed how crystals of sodium chloride. How Does Salt Dissociate In Water.
From www.koyuncusalt.com
Why Does Salt Dissolve In Water? How to Separate Them Back? Salt How Does Salt Dissociate In Water The science of salt dissolving in water • salt water chemistry • learn how salt dissolves in water through the process of ionization,. We finally know in detail how salt dissolves in water. Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then. The negative areas of the water molecules surround the. The water molecules. How Does Salt Dissociate In Water.
From saylordotorg.github.io
Aqueous Solutions How Does Salt Dissociate In Water The negative areas of the water molecules surround the. The process of dissolving involves the breaking up of a solute (salt) into individual particles and dispersing them throughout a. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. Water molecules pulling apart the ions (sodium and chloride) in a. How Does Salt Dissociate In Water.
From schematiclistpact101.z22.web.core.windows.net
Salt Dissolving In Water Diagram How Does Salt Dissociate In Water The positive areas of the water molecules surround the negative chloride ions. We finally know in detail how salt dissolves in water. One common approach to melting the ice is to put some form of deicing salt on the surface. When salt is added to water, the positive and negative ions separate. The science of salt dissolving in water •. How Does Salt Dissociate In Water.
From wou.edu
CH150 Chapter 7 Solutions Chemistry How Does Salt Dissociate In Water The process of dissolving involves the breaking up of a solute (salt) into individual particles and dispersing them throughout a. The water molecules surround the ions and pull them. One common approach to melting the ice is to put some form of deicing salt on the surface. We finally know in detail how salt dissolves in water. The science of. How Does Salt Dissociate In Water.
From eduvik.in
Matter in Our Surroundings Class 9 Notes Science Chapter 1 Eduvik How Does Salt Dissociate In Water A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. The science of salt dissolving in water • salt water chemistry • learn how salt dissolves in water through the process of ionization,. One common approach to melting the ice is to put some form of deicing salt on the. How Does Salt Dissociate In Water.
From www.aquriousmind.com
Why does salt dissolve in water? AQuriousMind How Does Salt Dissociate In Water A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in. We finally know in detail how salt dissolves in water. The process of dissolving involves the breaking up of a solute (salt) into individual particles and dispersing them throughout a. The water molecules surround the ions and pull them. When. How Does Salt Dissociate In Water.
From www.slideserve.com
PPT Acid/Base Properties of Salts PowerPoint Presentation, free How Does Salt Dissociate In Water The positive areas of the water molecules surround the negative chloride ions. The science of salt dissolving in water • salt water chemistry • learn how salt dissolves in water through the process of ionization,. When salt is added to water, the positive and negative ions separate. The process of dissolving involves the breaking up of a solute (salt) into. How Does Salt Dissociate In Water.