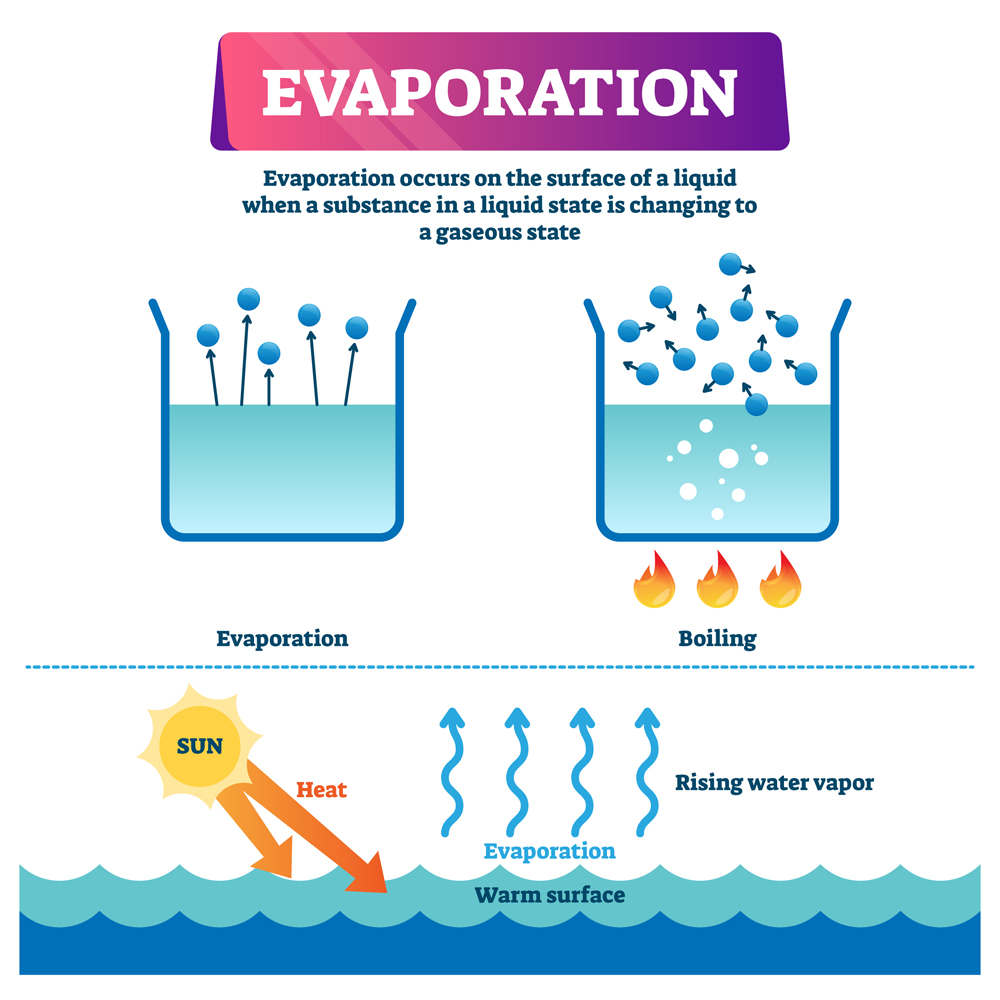
Evaporation Effects Temperature . Because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. Thermal physics 2.2 thermal properties & temperature evaporation. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Evaporation can take place at any temperature. Low humidity, on the other hand, can cause discomfort from excessive. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. It is also how liquid water enters. Scientists investigate how temperature affects how quickly a liquid evaporates to a gas. Since the kinetic energy of a molecule is proportional to its temperature, evaporation proceeds more quickly at higher temperatures. Condensation is the change of state. Temperature at which this occurs?
from www.scienceabc.com
Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. Since the kinetic energy of a molecule is proportional to its temperature, evaporation proceeds more quickly at higher temperatures. Thermal physics 2.2 thermal properties & temperature evaporation. Low humidity, on the other hand, can cause discomfort from excessive. It is also how liquid water enters. Temperature at which this occurs? Condensation is the change of state. Scientists investigate how temperature affects how quickly a liquid evaporates to a gas. Evaporation can take place at any temperature.
Why Does Water Evaporate At Room Temperature?
Evaporation Effects Temperature Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. It is also how liquid water enters. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. Since the kinetic energy of a molecule is proportional to its temperature, evaporation proceeds more quickly at higher temperatures. Condensation is the change of state. Evaporation can take place at any temperature. Temperature at which this occurs? Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Low humidity, on the other hand, can cause discomfort from excessive. Thermal physics 2.2 thermal properties & temperature evaporation. Scientists investigate how temperature affects how quickly a liquid evaporates to a gas.
From www.researchgate.net
(a) The effects of evaporation temperature on exergy efficiency and Evaporation Effects Temperature Low humidity, on the other hand, can cause discomfort from excessive. Temperature at which this occurs? Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. It is also how liquid water enters. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling. Evaporation Effects Temperature.
From www.researchgate.net
Effect of temperature on the evaporation curves. Full circles refer to Evaporation Effects Temperature Thermal physics 2.2 thermal properties & temperature evaporation. Scientists investigate how temperature affects how quickly a liquid evaporates to a gas. Low humidity, on the other hand, can cause discomfort from excessive. Temperature at which this occurs? Since the kinetic energy of a molecule is proportional to its temperature, evaporation proceeds more quickly at higher temperatures. Evaporation, process by which. Evaporation Effects Temperature.
From www.researchgate.net
The effect of temperature and height of the channel on the evaporation Evaporation Effects Temperature Thermal physics 2.2 thermal properties & temperature evaporation. Low humidity, on the other hand, can cause discomfort from excessive. Because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. It is also how liquid water enters. Temperature at which this occurs? Evaporation can take place at any temperature. Condensation is the. Evaporation Effects Temperature.
From www.researchgate.net
Effect of evaporation temperature Download Table Evaporation Effects Temperature Evaporation can take place at any temperature. Condensation is the change of state. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. Low humidity, on the other hand, can. Evaporation Effects Temperature.
From www.researchgate.net
Feedback diagram illustrating the effects of evaporite weathering on Evaporation Effects Temperature Evaporation can take place at any temperature. Low humidity, on the other hand, can cause discomfort from excessive. Condensation is the change of state. Because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. Temperature at which this occurs? Evaporation, process by which an element or compound transitions from its liquid. Evaporation Effects Temperature.
From www.researchgate.net
(a) The effects of evaporation temperature on exergy efficiency and Evaporation Effects Temperature Condensation is the change of state. Evaporation can take place at any temperature. Because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. Since the kinetic energy of a molecule is proportional to its temperature, evaporation proceeds more quickly at higher temperatures. Thermal physics 2.2 thermal properties & temperature evaporation. Temperature. Evaporation Effects Temperature.
From www.researchgate.net
Effect of evaporation temperature on net power generation and Evaporation Effects Temperature It is also how liquid water enters. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Scientists investigate how temperature affects how quickly a liquid evaporates to a gas. Condensation is the change of state. Low humidity, on the other hand, can cause discomfort from excessive. Evaporation can. Evaporation Effects Temperature.
From www.cgpplus.co.uk
How Does Temperature Affect Evaporation? (Year 4) CGP Plus Evaporation Effects Temperature Since the kinetic energy of a molecule is proportional to its temperature, evaporation proceeds more quickly at higher temperatures. It is also how liquid water enters. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Evaporation can take place at any temperature. Evaporation is the conversion of a. Evaporation Effects Temperature.
From www.youtube.com
Study of Effect of Temperature on Rate of Evaporation. YouTube Evaporation Effects Temperature Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Scientists investigate how temperature affects how quickly a liquid evaporates to a gas. It is also how liquid water enters. Condensation is the change of state. Since the kinetic energy of a molecule is proportional to its temperature, evaporation. Evaporation Effects Temperature.
From www.researchgate.net
a Schematic illustration of water evaporation process and mechanism. b Evaporation Effects Temperature Because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Evaporation can take place at any temperature. Since the kinetic energy of a molecule is proportional to its temperature, evaporation proceeds more quickly at. Evaporation Effects Temperature.
From www.researchgate.net
(PDF) Effects of mechanical aeration on evaporation rate, water temperature Evaporation Effects Temperature Thermal physics 2.2 thermal properties & temperature evaporation. Because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. Scientists investigate how temperature affects how quickly a liquid evaporates to a gas. Low humidity, on the other hand, can cause discomfort from excessive. Evaporation can take place at any temperature. Evaporation, process. Evaporation Effects Temperature.
From www.researchgate.net
Effect of evaporation temperature on supply temperature under different Evaporation Effects Temperature Evaporation can take place at any temperature. Temperature at which this occurs? Low humidity, on the other hand, can cause discomfort from excessive. It is also how liquid water enters. Since the kinetic energy of a molecule is proportional to its temperature, evaporation proceeds more quickly at higher temperatures. Evaporation is the conversion of a liquid to its vapor below. Evaporation Effects Temperature.
From www.researchgate.net
Effect of evaporation temperature on the proportion of exergy Evaporation Effects Temperature Low humidity, on the other hand, can cause discomfort from excessive. Thermal physics 2.2 thermal properties & temperature evaporation. Evaporation can take place at any temperature. It is also how liquid water enters. Since the kinetic energy of a molecule is proportional to its temperature, evaporation proceeds more quickly at higher temperatures. Because evaporation is inhibited by high humidity, we. Evaporation Effects Temperature.
From www.researchgate.net
Effects of PDA evaporation temperature on permeance and rejection (a Evaporation Effects Temperature Low humidity, on the other hand, can cause discomfort from excessive. Temperature at which this occurs? Thermal physics 2.2 thermal properties & temperature evaporation. Condensation is the change of state. Scientists investigate how temperature affects how quickly a liquid evaporates to a gas. Because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity. Evaporation Effects Temperature.
From www.researchgate.net
Effects of the evaporation temperature on the system performance (A Evaporation Effects Temperature Temperature at which this occurs? Since the kinetic energy of a molecule is proportional to its temperature, evaporation proceeds more quickly at higher temperatures. Because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. Condensation is the change of state. Scientists investigate how temperature affects how quickly a liquid evaporates to. Evaporation Effects Temperature.
From www.slideshare.net
Evaporation Evaporation Effects Temperature Scientists investigate how temperature affects how quickly a liquid evaporates to a gas. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. Temperature at which this occurs? It is. Evaporation Effects Temperature.
From www.youtube.com
evaporation, Factors affecting evaporation, CBSE 9, Temperature Evaporation Effects Temperature Since the kinetic energy of a molecule is proportional to its temperature, evaporation proceeds more quickly at higher temperatures. Evaporation can take place at any temperature. Because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. It is also how liquid water enters. Evaporation, process by which an element or compound. Evaporation Effects Temperature.
From www.slideserve.com
PPT Intermolecular Attractions & the Properties of Liquids & Solids Evaporation Effects Temperature Low humidity, on the other hand, can cause discomfort from excessive. Evaporation can take place at any temperature. Scientists investigate how temperature affects how quickly a liquid evaporates to a gas. Since the kinetic energy of a molecule is proportional to its temperature, evaporation proceeds more quickly at higher temperatures. Evaporation is the conversion of a liquid to its vapor. Evaporation Effects Temperature.
From socratic.org
How does temperature affect the atmosphere and cause weather? Socratic Evaporation Effects Temperature It is also how liquid water enters. Since the kinetic energy of a molecule is proportional to its temperature, evaporation proceeds more quickly at higher temperatures. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity. Evaporation Effects Temperature.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Evaporation Effects Temperature Evaporation can take place at any temperature. It is also how liquid water enters. Condensation is the change of state. Scientists investigate how temperature affects how quickly a liquid evaporates to a gas. Because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. Evaporation, process by which an element or compound. Evaporation Effects Temperature.
From www.researchgate.net
Effect of evaporation temperature on performances of dual pressure Evaporation Effects Temperature Low humidity, on the other hand, can cause discomfort from excessive. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Evaporation can take place at any temperature. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Since the kinetic energy. Evaporation Effects Temperature.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Evaporation Effects Temperature It is also how liquid water enters. Condensation is the change of state. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Low humidity, on the other hand, can cause discomfort from excessive. Since the kinetic energy of a molecule is proportional to its temperature, evaporation proceeds more quickly at higher temperatures.. Evaporation Effects Temperature.
From www.researchgate.net
Effect of evaporation temperature on mass flow rate of various organic Evaporation Effects Temperature Because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. Low humidity, on the other hand, can cause discomfort from excessive. Scientists investigate how temperature affects how quickly a liquid evaporates to a gas. Since the kinetic energy of a molecule is proportional to its temperature, evaporation proceeds more quickly at. Evaporation Effects Temperature.
From www.researchgate.net
Effect of evaporation temperature on signal intensity. Conditions Bi Evaporation Effects Temperature It is also how liquid water enters. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Thermal physics 2.2 thermal properties & temperature evaporation. Because evaporation is inhibited by high humidity,. Evaporation Effects Temperature.
From www.researchgate.net
Variation of GOR with evaporation temperature in last effect Evaporation Effects Temperature Since the kinetic energy of a molecule is proportional to its temperature, evaporation proceeds more quickly at higher temperatures. Condensation is the change of state. Low humidity, on the other hand, can cause discomfort from excessive. Evaporation can take place at any temperature. It is also how liquid water enters. Because evaporation is inhibited by high humidity, we feel hotter. Evaporation Effects Temperature.
From www.researchgate.net
Effects of temperature and ethanol concentration on ethanol Evaporation Effects Temperature Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. It is also. Evaporation Effects Temperature.
From www.researchgate.net
(a) The effects of evaporation temperature (XXmust be read on the Evaporation Effects Temperature Temperature at which this occurs? Thermal physics 2.2 thermal properties & temperature evaporation. Low humidity, on the other hand, can cause discomfort from excessive. It is also how liquid water enters. Condensation is the change of state. Because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. Evaporation, process by which. Evaporation Effects Temperature.
From brainly.in
how does evaporation cause cooling?? Brainly.in Evaporation Effects Temperature Thermal physics 2.2 thermal properties & temperature evaporation. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Temperature at which this occurs? Low humidity, on the other hand, can cause discomfort from excessive. Evaporation can take place at any temperature. Scientists investigate how temperature affects how quickly a. Evaporation Effects Temperature.
From climate.nasa.gov
Steamy Relationships How Atmospheric Water Vapor Amplifies Earth's Evaporation Effects Temperature Since the kinetic energy of a molecule is proportional to its temperature, evaporation proceeds more quickly at higher temperatures. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Low humidity, on the other hand, can cause discomfort from excessive. Thermal physics 2.2 thermal properties & temperature evaporation. Because evaporation is inhibited by. Evaporation Effects Temperature.
From www.teachoo.com
How does Evaporation cause cooling? Explain (with Examples) Teachoo Evaporation Effects Temperature Condensation is the change of state. Thermal physics 2.2 thermal properties & temperature evaporation. Evaporation can take place at any temperature. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Scientists investigate how temperature affects how quickly a liquid evaporates to a gas. Evaporation is the conversion of. Evaporation Effects Temperature.
From how-things-work-science-projects.com
How do Temperature and Wind affect Evaporation? How Things Work Evaporation Effects Temperature Because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. Temperature at which this occurs? Thermal physics 2.2 thermal properties & temperature evaporation. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Scientists investigate how temperature affects how quickly. Evaporation Effects Temperature.
From www.researchgate.net
Effect of evaporation temperature on the crosssection and surface Evaporation Effects Temperature Low humidity, on the other hand, can cause discomfort from excessive. Because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. Thermal physics 2.2 thermal properties & temperature evaporation. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Scientists investigate how temperature affects. Evaporation Effects Temperature.
From www.researchgate.net
For two different evaporation temperatures the dependence of the Evaporation Effects Temperature Scientists investigate how temperature affects how quickly a liquid evaporates to a gas. Because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. Temperature at which this occurs? Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Evaporation is. Evaporation Effects Temperature.
From learningschooltrkesp5v.z22.web.core.windows.net
Water Evaporation Rate By Temperature Evaporation Effects Temperature Since the kinetic energy of a molecule is proportional to its temperature, evaporation proceeds more quickly at higher temperatures. It is also how liquid water enters. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Temperature at which this occurs? Evaporation, process by which an element or compound transitions from its liquid. Evaporation Effects Temperature.
From climate.nasa.gov
Steamy Relationships How Atmospheric Water Vapor Amplifies Earth's Evaporation Effects Temperature Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below its boiling temperature. Since the kinetic energy of a molecule is proportional to its temperature, evaporation proceeds more quickly at higher temperatures. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Condensation is the. Evaporation Effects Temperature.