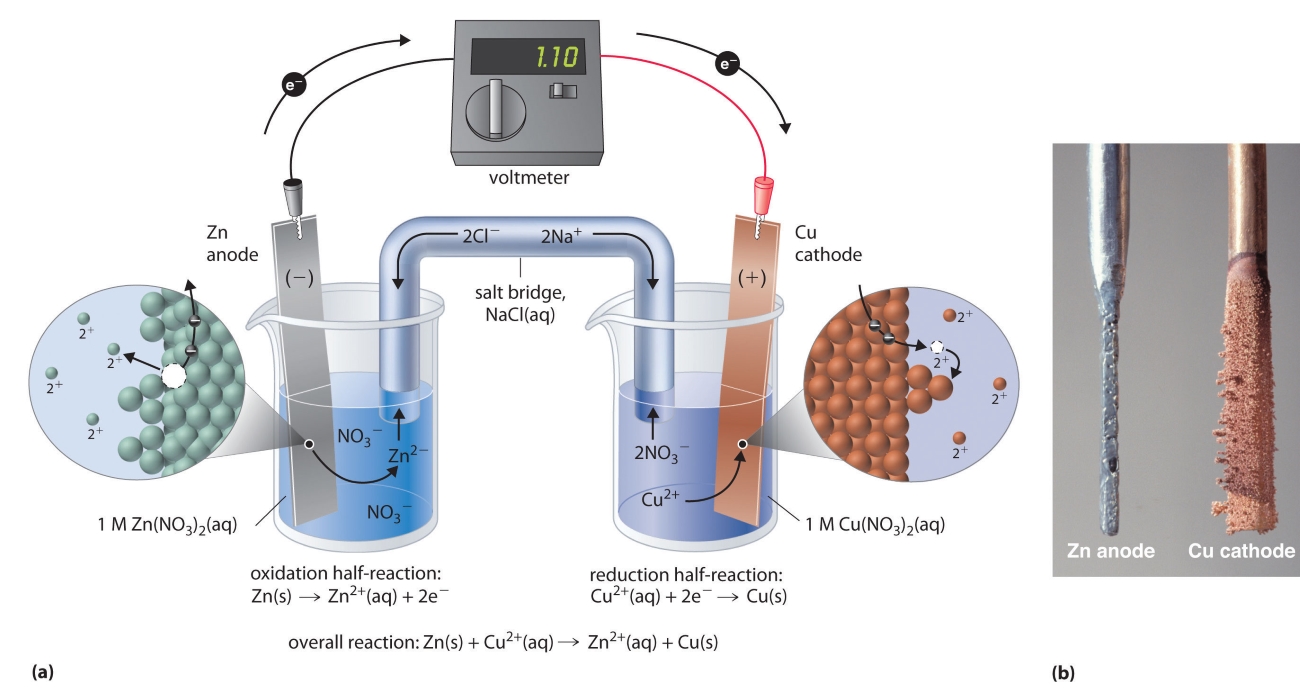
Galvanic Cell Quizlet . What is a galvanic cell? A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\ (δg < 0\)) to generate electricity. The zinc electrode loses mass and the zinc electrode is the cathode. A chemical system that produces an electric current from a spontaneous redox reaction. How can you tell if a reaction is. The zinc electrode gains mass and the zinc electrode is the anode. Transform chemical potential energy into electrical energy via a spontaneous redox reaction to produce a voltage that results. Zn (s) + cu 2+ (aq) ® cu (s) + zn 2+ (aq) when the cell is running spontaneously,. Consider the galvanic cell shown in this animation.
from exomnwzaf.blob.core.windows.net
What is a galvanic cell? The zinc electrode gains mass and the zinc electrode is the anode. The zinc electrode loses mass and the zinc electrode is the cathode. How can you tell if a reaction is. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\ (δg < 0\)) to generate electricity. Transform chemical potential energy into electrical energy via a spontaneous redox reaction to produce a voltage that results. Consider the galvanic cell shown in this animation. Zn (s) + cu 2+ (aq) ® cu (s) + zn 2+ (aq) when the cell is running spontaneously,. A chemical system that produces an electric current from a spontaneous redox reaction.
What Is The Electrochemical Equivalent at Marcus Evenson blog
Galvanic Cell Quizlet Consider the galvanic cell shown in this animation. Zn (s) + cu 2+ (aq) ® cu (s) + zn 2+ (aq) when the cell is running spontaneously,. How can you tell if a reaction is. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\ (δg < 0\)) to generate electricity. The zinc electrode loses mass and the zinc electrode is the cathode. Transform chemical potential energy into electrical energy via a spontaneous redox reaction to produce a voltage that results. A chemical system that produces an electric current from a spontaneous redox reaction. The zinc electrode gains mass and the zinc electrode is the anode. Consider the galvanic cell shown in this animation. What is a galvanic cell?
From www.thinkswap.com
Galvanic Cell FIA1163 Professional Writing UCTS Thinkswap Galvanic Cell Quizlet Transform chemical potential energy into electrical energy via a spontaneous redox reaction to produce a voltage that results. Consider the galvanic cell shown in this animation. How can you tell if a reaction is. What is a galvanic cell? A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\ (δg < 0\)) to generate electricity. A. Galvanic Cell Quizlet.
From pubhtml5.com
galvanic cell cheera2008 पेज पर 1 52 ऑनलाइन पीडीएफ फ्लिप करा Galvanic Cell Quizlet The zinc electrode loses mass and the zinc electrode is the cathode. Zn (s) + cu 2+ (aq) ® cu (s) + zn 2+ (aq) when the cell is running spontaneously,. What is a galvanic cell? A chemical system that produces an electric current from a spontaneous redox reaction. Consider the galvanic cell shown in this animation. The zinc electrode. Galvanic Cell Quizlet.
From quizlet.com
General chemistry 9(a) Galvanic Cells Diagram Quizlet Galvanic Cell Quizlet The zinc electrode loses mass and the zinc electrode is the cathode. How can you tell if a reaction is. Zn (s) + cu 2+ (aq) ® cu (s) + zn 2+ (aq) when the cell is running spontaneously,. What is a galvanic cell? A chemical system that produces an electric current from a spontaneous redox reaction. A galvanic (voltaic). Galvanic Cell Quizlet.
From guidemanualcodas.z1.web.core.windows.net
Diagram Of Voltaic Cell Galvanic Cell Quizlet How can you tell if a reaction is. A chemical system that produces an electric current from a spontaneous redox reaction. What is a galvanic cell? Transform chemical potential energy into electrical energy via a spontaneous redox reaction to produce a voltage that results. Zn (s) + cu 2+ (aq) ® cu (s) + zn 2+ (aq) when the cell. Galvanic Cell Quizlet.
From socratic.org
How can a galvanic cell an electrolytic cell? Socratic Galvanic Cell Quizlet Zn (s) + cu 2+ (aq) ® cu (s) + zn 2+ (aq) when the cell is running spontaneously,. Consider the galvanic cell shown in this animation. A chemical system that produces an electric current from a spontaneous redox reaction. How can you tell if a reaction is. What is a galvanic cell? The zinc electrode loses mass and the. Galvanic Cell Quizlet.
From quizlet.com
Galvanic Cells Diagram Quizlet Galvanic Cell Quizlet Zn (s) + cu 2+ (aq) ® cu (s) + zn 2+ (aq) when the cell is running spontaneously,. The zinc electrode gains mass and the zinc electrode is the anode. A chemical system that produces an electric current from a spontaneous redox reaction. What is a galvanic cell? Transform chemical potential energy into electrical energy via a spontaneous redox. Galvanic Cell Quizlet.
From hxectqqav.blob.core.windows.net
Electrolytic Electrode Charge at Keith Sullivan blog Galvanic Cell Quizlet The zinc electrode gains mass and the zinc electrode is the anode. Zn (s) + cu 2+ (aq) ® cu (s) + zn 2+ (aq) when the cell is running spontaneously,. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\ (δg < 0\)) to generate electricity. A chemical system that produces an electric current from. Galvanic Cell Quizlet.
From quizlet.com
chemistry sem ii redox (galvanic cell diagram) Diagram Quizlet Galvanic Cell Quizlet The zinc electrode gains mass and the zinc electrode is the anode. What is a galvanic cell? Zn (s) + cu 2+ (aq) ® cu (s) + zn 2+ (aq) when the cell is running spontaneously,. How can you tell if a reaction is. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\ (δg <. Galvanic Cell Quizlet.
From quizlet.com
Label the zinc/copper galvanic cell Diagram Quizlet Galvanic Cell Quizlet Zn (s) + cu 2+ (aq) ® cu (s) + zn 2+ (aq) when the cell is running spontaneously,. The zinc electrode loses mass and the zinc electrode is the cathode. The zinc electrode gains mass and the zinc electrode is the anode. How can you tell if a reaction is. A chemical system that produces an electric current from. Galvanic Cell Quizlet.
From www.coursehero.com
[Solved] Galvanic Cells Sketch the galvanic cells based on the Galvanic Cell Quizlet A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\ (δg < 0\)) to generate electricity. How can you tell if a reaction is. What is a galvanic cell? Consider the galvanic cell shown in this animation. The zinc electrode loses mass and the zinc electrode is the cathode. A chemical system that produces an electric. Galvanic Cell Quizlet.
From courses.lumenlearning.com
Galvanic Cells General Chemistry Galvanic Cell Quizlet A chemical system that produces an electric current from a spontaneous redox reaction. What is a galvanic cell? A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\ (δg < 0\)) to generate electricity. How can you tell if a reaction is. Zn (s) + cu 2+ (aq) ® cu (s) + zn 2+ (aq) when. Galvanic Cell Quizlet.
From quizlet.com
Sketch and label the parts of a galvanic cell. Quizlet Galvanic Cell Quizlet Transform chemical potential energy into electrical energy via a spontaneous redox reaction to produce a voltage that results. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\ (δg < 0\)) to generate electricity. Zn (s) + cu 2+ (aq) ® cu (s) + zn 2+ (aq) when the cell is running spontaneously,. Consider the galvanic. Galvanic Cell Quizlet.
From hxebwxopr.blob.core.windows.net
Example Of Galvanic Cell And Electrolytic Cell at Tunstall blog Galvanic Cell Quizlet A chemical system that produces an electric current from a spontaneous redox reaction. The zinc electrode loses mass and the zinc electrode is the cathode. Transform chemical potential energy into electrical energy via a spontaneous redox reaction to produce a voltage that results. Zn (s) + cu 2+ (aq) ® cu (s) + zn 2+ (aq) when the cell is. Galvanic Cell Quizlet.
From quizlet.com
Draw a diagram of a simple galvanic cell. Quizlet Galvanic Cell Quizlet How can you tell if a reaction is. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\ (δg < 0\)) to generate electricity. The zinc electrode loses mass and the zinc electrode is the cathode. Transform chemical potential energy into electrical energy via a spontaneous redox reaction to produce a voltage that results. A chemical. Galvanic Cell Quizlet.
From exomnwzaf.blob.core.windows.net
What Is The Electrochemical Equivalent at Marcus Evenson blog Galvanic Cell Quizlet What is a galvanic cell? How can you tell if a reaction is. A chemical system that produces an electric current from a spontaneous redox reaction. Zn (s) + cu 2+ (aq) ® cu (s) + zn 2+ (aq) when the cell is running spontaneously,. The zinc electrode loses mass and the zinc electrode is the cathode. Consider the galvanic. Galvanic Cell Quizlet.
From circuitagolopetzin.z4.web.core.windows.net
What Forms At The Cathode During Electrolysis Galvanic Cell Quizlet The zinc electrode loses mass and the zinc electrode is the cathode. A chemical system that produces an electric current from a spontaneous redox reaction. Consider the galvanic cell shown in this animation. How can you tell if a reaction is. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\ (δg < 0\)) to generate. Galvanic Cell Quizlet.
From socratic.org
Two halfcells in a galvanic cell consist of one iron (Fe(s)) electrode Galvanic Cell Quizlet The zinc electrode gains mass and the zinc electrode is the anode. A chemical system that produces an electric current from a spontaneous redox reaction. Consider the galvanic cell shown in this animation. What is a galvanic cell? Transform chemical potential energy into electrical energy via a spontaneous redox reaction to produce a voltage that results. How can you tell. Galvanic Cell Quizlet.
From www.chemistrysources.com
الخلية الجلفانية Galvanic Cell مصادر الكيمياء Galvanic Cell Quizlet Consider the galvanic cell shown in this animation. The zinc electrode gains mass and the zinc electrode is the anode. The zinc electrode loses mass and the zinc electrode is the cathode. Transform chemical potential energy into electrical energy via a spontaneous redox reaction to produce a voltage that results. How can you tell if a reaction is. A chemical. Galvanic Cell Quizlet.
From quizlet.com
Galvanic Cell vs Electrolytic Cell Diagram Quizlet Galvanic Cell Quizlet Zn (s) + cu 2+ (aq) ® cu (s) + zn 2+ (aq) when the cell is running spontaneously,. The zinc electrode gains mass and the zinc electrode is the anode. How can you tell if a reaction is. A chemical system that produces an electric current from a spontaneous redox reaction. What is a galvanic cell? Consider the galvanic. Galvanic Cell Quizlet.
From www.studocu.com
Galvanic Cells Galvanic Cells Definitions Spontaneous = chemical Galvanic Cell Quizlet The zinc electrode gains mass and the zinc electrode is the anode. A chemical system that produces an electric current from a spontaneous redox reaction. What is a galvanic cell? How can you tell if a reaction is. Zn (s) + cu 2+ (aq) ® cu (s) + zn 2+ (aq) when the cell is running spontaneously,. The zinc electrode. Galvanic Cell Quizlet.
From www.shutterstock.com
Daniell Cell Best Example Galvanic Cell Stock Vector (Royalty Free Galvanic Cell Quizlet The zinc electrode gains mass and the zinc electrode is the anode. Zn (s) + cu 2+ (aq) ® cu (s) + zn 2+ (aq) when the cell is running spontaneously,. A chemical system that produces an electric current from a spontaneous redox reaction. Transform chemical potential energy into electrical energy via a spontaneous redox reaction to produce a voltage. Galvanic Cell Quizlet.
From quizlet.com
GALVANIC CELL Diagram Quizlet Galvanic Cell Quizlet Consider the galvanic cell shown in this animation. The zinc electrode loses mass and the zinc electrode is the cathode. A chemical system that produces an electric current from a spontaneous redox reaction. What is a galvanic cell? A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\ (δg < 0\)) to generate electricity. The zinc. Galvanic Cell Quizlet.
From learningkurugon1.z22.web.core.windows.net
Electrochemical Cell Questions And Answers Galvanic Cell Quizlet Consider the galvanic cell shown in this animation. What is a galvanic cell? The zinc electrode gains mass and the zinc electrode is the anode. Zn (s) + cu 2+ (aq) ® cu (s) + zn 2+ (aq) when the cell is running spontaneously,. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\ (δg <. Galvanic Cell Quizlet.
From quizlet.com
galvanic cell Diagram Quizlet Galvanic Cell Quizlet The zinc electrode loses mass and the zinc electrode is the cathode. What is a galvanic cell? Zn (s) + cu 2+ (aq) ® cu (s) + zn 2+ (aq) when the cell is running spontaneously,. Transform chemical potential energy into electrical energy via a spontaneous redox reaction to produce a voltage that results. Consider the galvanic cell shown in. Galvanic Cell Quizlet.