Medical Device Samples . The updated methodology guide is aimed at manufacturers, research structures, project leaders and healthcare professionals involved. As part of design and development validation, the organization shall perform clinical evaluations or performance evaluations of the medical device in accordance with applicable regulatory. Blood glucose meters, hearing aids, and nebulisers. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) establish the. Medical devices are products or equipment intended for a medical purpose. Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. In the european union (eu) they must undergo a conformity. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. Mri scanners, pacemakers, and robotic surgical systems.
from laegemiddelstyrelsen.dk
Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) establish the. In the european union (eu) they must undergo a conformity. Mri scanners, pacemakers, and robotic surgical systems. As part of design and development validation, the organization shall perform clinical evaluations or performance evaluations of the medical device in accordance with applicable regulatory. Medical devices are products or equipment intended for a medical purpose. Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. Blood glucose meters, hearing aids, and nebulisers. The updated methodology guide is aimed at manufacturers, research structures, project leaders and healthcare professionals involved.
Medical devices
Medical Device Samples In the european union (eu) they must undergo a conformity. Mri scanners, pacemakers, and robotic surgical systems. The updated methodology guide is aimed at manufacturers, research structures, project leaders and healthcare professionals involved. Blood glucose meters, hearing aids, and nebulisers. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) establish the. In the european union (eu) they must undergo a conformity. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. Medical devices are products or equipment intended for a medical purpose. Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. As part of design and development validation, the organization shall perform clinical evaluations or performance evaluations of the medical device in accordance with applicable regulatory.
From barcode-labels.com
Medical Device Labels Electronic Imaging Materials Medical Device Samples The updated methodology guide is aimed at manufacturers, research structures, project leaders and healthcare professionals involved. As part of design and development validation, the organization shall perform clinical evaluations or performance evaluations of the medical device in accordance with applicable regulatory. In the european union (eu) they must undergo a conformity. Mri scanners, pacemakers, and robotic surgical systems. Blood glucose. Medical Device Samples.
From senzagen.com
Overview Biological Evaluation of Medical Devices Senzagen Medical Device Samples Blood glucose meters, hearing aids, and nebulisers. In the european union (eu) they must undergo a conformity. Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. As part of design and development validation, the organization shall perform clinical evaluations or performance evaluations of the medical device in accordance with. Medical Device Samples.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Medical Device Samples In the european union (eu) they must undergo a conformity. Mri scanners, pacemakers, and robotic surgical systems. As part of design and development validation, the organization shall perform clinical evaluations or performance evaluations of the medical device in accordance with applicable regulatory. Medical devices are products or equipment intended for a medical purpose. Regulation (eu) 2017/745 on medical devices (mdr). Medical Device Samples.
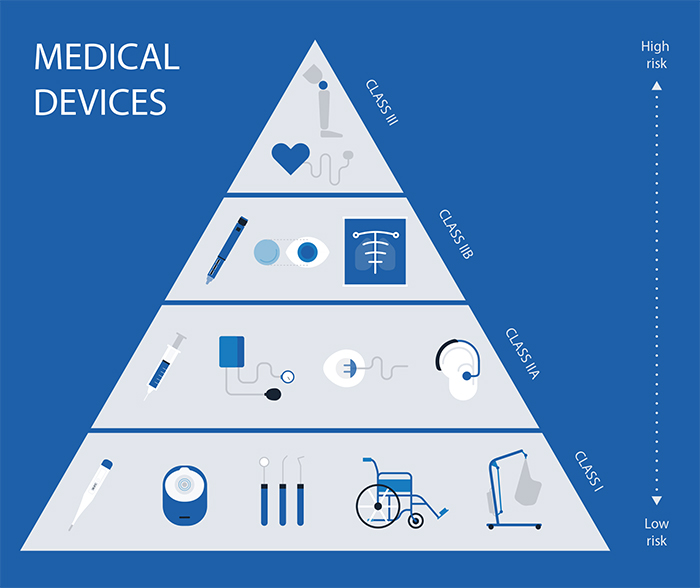
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Samples In the european union (eu) they must undergo a conformity. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) establish the. Medical devices are products or equipment intended for a medical purpose. The updated methodology guide is aimed at manufacturers, research structures, project leaders and healthcare professionals involved. Determine if your. Medical Device Samples.
From talema.com
An Introduction to Medical Electrical Devices The Talema Group Medical Device Samples The updated methodology guide is aimed at manufacturers, research structures, project leaders and healthcare professionals involved. As part of design and development validation, the organization shall perform clinical evaluations or performance evaluations of the medical device in accordance with applicable regulatory. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug. Medical Device Samples.
From laegemiddelstyrelsen.dk
Medical devices Medical Device Samples Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) establish the. The updated methodology guide is aimed at manufacturers, research structures, project leaders and healthcare professionals involved. Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. Blood glucose meters, hearing. Medical Device Samples.
From www.conveyco.com
Medical Device & Pharmaceutical Products Medical Device Samples Mri scanners, pacemakers, and robotic surgical systems. Blood glucose meters, hearing aids, and nebulisers. As part of design and development validation, the organization shall perform clinical evaluations or performance evaluations of the medical device in accordance with applicable regulatory. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic. Medical Device Samples.
From www.ecobliss-pharma.com
Medical Device Packaging Ecobliss Pharmaceutical Packaging Medical Device Samples Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. In the european union (eu) they must undergo a conformity. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) establish the. As part of design and development validation, the organization shall. Medical Device Samples.
From angelanjohnson.com
Medical Devices Angela N Johnson Medical Device Samples The updated methodology guide is aimed at manufacturers, research structures, project leaders and healthcare professionals involved. Medical devices are products or equipment intended for a medical purpose. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. Blood glucose meters, hearing aids, and nebulisers. Mri scanners, pacemakers, and. Medical Device Samples.
From www.cncprototype-machining.com
Medical Device Mock Model Samples Rapid Prototype CNC Process Medical Device Samples Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. The updated methodology guide is aimed at manufacturers, research structures, project leaders and healthcare professionals involved. As part of design and development validation, the organization shall perform clinical evaluations or performance evaluations of the medical device in accordance with applicable. Medical Device Samples.
From klanjwrju.blob.core.windows.net
Fda Medical Device Labeling Guidance at Michael Crawford blog Medical Device Samples Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) establish the. Blood glucose meters, hearing aids, and nebulisers. The updated methodology guide is aimed at manufacturers, research structures, project leaders and healthcare professionals involved. Determine if your product meets the definition of a medical device per section 201 (h) of the. Medical Device Samples.
From www.greenlight.guru
8 Tips Before Calculating Sample Size in Medical Device Clinical Studies Medical Device Samples Mri scanners, pacemakers, and robotic surgical systems. As part of design and development validation, the organization shall perform clinical evaluations or performance evaluations of the medical device in accordance with applicable regulatory. In the european union (eu) they must undergo a conformity. Determine if your product meets the definition of a medical device per section 201 (h) of the food,. Medical Device Samples.
From www.dreamstime.com
Samples from Medical Tests are Loaded into the Device Stock Photo Medical Device Samples Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) establish the. Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. Mri scanners, pacemakers, and robotic surgical systems. As part of design and development validation, the organization shall perform clinical evaluations. Medical Device Samples.
From www.campoly.com
Material selection in medical devices Medical Device Samples Mri scanners, pacemakers, and robotic surgical systems. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) establish the. Medical devices are products or equipment intended for a medical purpose. The updated methodology guide is aimed at manufacturers, research structures, project leaders and healthcare professionals involved. Determine if your product meets the. Medical Device Samples.
From www.massdevice.com
An expert's guide to developing medical devices MassDevice Medical Device Samples Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) establish the. The updated methodology guide is aimed at manufacturers, research structures, project leaders and healthcare professionals involved. In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose. Determine if your. Medical Device Samples.
From lifechanginginnovation.org
What is a Medical Device? Life Changing Innovation Medical Device Samples As part of design and development validation, the organization shall perform clinical evaluations or performance evaluations of the medical device in accordance with applicable regulatory. The updated methodology guide is aimed at manufacturers, research structures, project leaders and healthcare professionals involved. In the european union (eu) they must undergo a conformity. Medical devices are used in many diverse settings, for. Medical Device Samples.
From cohenhealthcarelaw.com
Understanding the Medical Device Classification and Approval Process Medical Device Samples Blood glucose meters, hearing aids, and nebulisers. Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. In the european union (eu) they must undergo a conformity. The updated methodology guide is aimed at manufacturers, research structures, project leaders and healthcare professionals involved. Mri scanners, pacemakers, and robotic surgical systems.. Medical Device Samples.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Samples Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. Blood glucose meters, hearing aids, and nebulisers. Mri scanners, pacemakers, and robotic surgical systems. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) establish the. In the european union (eu) they. Medical Device Samples.
From trinsicanimation.com
Medical Device Samples Trinsic Animation Medical Device Samples Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) establish the. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. The updated methodology guide is aimed at manufacturers, research structures, project leaders and healthcare professionals involved. Medical devices. Medical Device Samples.
From www.alamy.com
samples from medical tests are loaded into the device Stock Photo Alamy Medical Device Samples Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. Mri scanners, pacemakers, and robotic surgical systems. Medical devices are products or equipment intended for a medical purpose. The updated methodology guide is aimed at manufacturers, research structures, project leaders and healthcare professionals involved. Determine if your product meets the. Medical Device Samples.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Medical Device Samples Mri scanners, pacemakers, and robotic surgical systems. Blood glucose meters, hearing aids, and nebulisers. The updated methodology guide is aimed at manufacturers, research structures, project leaders and healthcare professionals involved. Medical devices are products or equipment intended for a medical purpose. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) establish. Medical Device Samples.
From www.conceptdraw.com
Medical Illustrations Solution Medical Device Samples As part of design and development validation, the organization shall perform clinical evaluations or performance evaluations of the medical device in accordance with applicable regulatory. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. Medical devices are products or equipment intended for a medical purpose. The updated. Medical Device Samples.
From www.greenlight.guru
Sample Size Calculation for Medical Device Studies Medical Device Samples The updated methodology guide is aimed at manufacturers, research structures, project leaders and healthcare professionals involved. Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. Medical devices are products or equipment intended for a medical purpose. Blood glucose meters, hearing aids, and nebulisers. In the european union (eu) they. Medical Device Samples.
From mavink.com
Types Of Medical Devices List Medical Device Samples As part of design and development validation, the organization shall perform clinical evaluations or performance evaluations of the medical device in accordance with applicable regulatory. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) establish the. Blood glucose meters, hearing aids, and nebulisers. In the european union (eu) they must undergo. Medical Device Samples.
From elisilac.com
What Is A Medical Device? Examples Elis Ilac Medical Device Samples Blood glucose meters, hearing aids, and nebulisers. As part of design and development validation, the organization shall perform clinical evaluations or performance evaluations of the medical device in accordance with applicable regulatory. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) establish the. Mri scanners, pacemakers, and robotic surgical systems. The. Medical Device Samples.
From elisilac.com
What Is A Medical Device? Examples Elis Ilac Medical Device Samples Medical devices are products or equipment intended for a medical purpose. Mri scanners, pacemakers, and robotic surgical systems. In the european union (eu) they must undergo a conformity. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) establish the. Blood glucose meters, hearing aids, and nebulisers. The updated methodology guide is. Medical Device Samples.
From operonstrategist.com
Medical Device Design & Development FAQ Operon Strategist Medical Device Samples Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. As part of design and development validation, the organization shall perform clinical evaluations or performance evaluations of. Medical Device Samples.
From www.youtube.com
diagnostic medical devices in daily uses / diagnostic medical equipment Medical Device Samples Medical devices are products or equipment intended for a medical purpose. As part of design and development validation, the organization shall perform clinical evaluations or performance evaluations of the medical device in accordance with applicable regulatory. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. In the. Medical Device Samples.
From medicaldevicehq.com
Different classifications rules for medical device software An Medical Device Samples Blood glucose meters, hearing aids, and nebulisers. As part of design and development validation, the organization shall perform clinical evaluations or performance evaluations of the medical device in accordance with applicable regulatory. Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. Determine if your product meets the definition of. Medical Device Samples.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Medical Device Samples Medical devices are products or equipment intended for a medical purpose. Mri scanners, pacemakers, and robotic surgical systems. In the european union (eu) they must undergo a conformity. Medical devices are used in many diverse settings, for example, by laypersons at home, by paramedical staff and clinicians in. Blood glucose meters, hearing aids, and nebulisers. As part of design and. Medical Device Samples.
From legacymedsearch.com
11 Most Innovative Medical Devices of 2017 Legacy MedSearch Medical Medical Device Samples As part of design and development validation, the organization shall perform clinical evaluations or performance evaluations of the medical device in accordance with applicable regulatory. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) establish the. The updated methodology guide is aimed at manufacturers, research structures, project leaders and healthcare professionals. Medical Device Samples.
From gdfeipin.com
implantablemedicaldevicesmedical GdfeiPin Medical Device Samples Mri scanners, pacemakers, and robotic surgical systems. The updated methodology guide is aimed at manufacturers, research structures, project leaders and healthcare professionals involved. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. In the european union (eu) they must undergo a conformity. As part of design and. Medical Device Samples.
From medium.com
The 3 FDA medical device classes [differences and examples explained Medical Device Samples Medical devices are products or equipment intended for a medical purpose. Mri scanners, pacemakers, and robotic surgical systems. The updated methodology guide is aimed at manufacturers, research structures, project leaders and healthcare professionals involved. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) establish the. Determine if your product meets the. Medical Device Samples.
From www.dreamstime.com
Samples from Medical Tests are Loaded into the Device Stock Photo Medical Device Samples Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) establish the. As part of design and development validation, the organization shall perform clinical evaluations or performance evaluations of the medical device in accordance with applicable regulatory. Medical devices are used in many diverse settings, for example, by laypersons at home, by. Medical Device Samples.
From www.conceptdraw.com
Medical devices Medical Device Samples Mri scanners, pacemakers, and robotic surgical systems. Regulation (eu) 2017/745 on medical devices (mdr) and regulation (eu) 2017/746 on in vitro diagnostic medical devices (ivdr) establish the. In the european union (eu) they must undergo a conformity. Blood glucose meters, hearing aids, and nebulisers. Determine if your product meets the definition of a medical device per section 201 (h) of. Medical Device Samples.