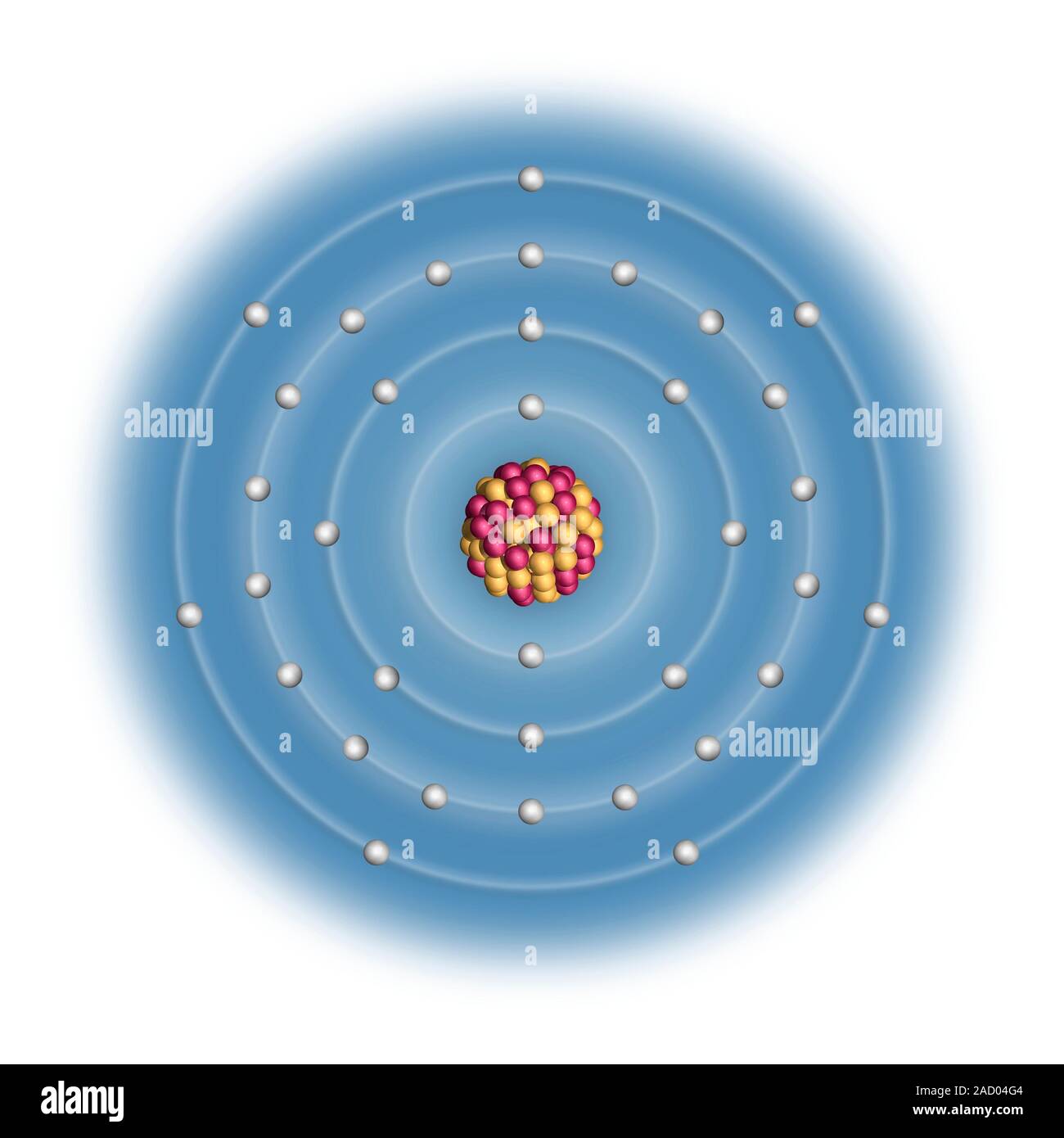
Bromine Electron Configuration Long Form . This can be shortened to [ar]4s23d104p5. Bromine (br) is a chemical element. It evaporates readily and forms a colored gas. The atomic number of bromine is 35. The full version of this is: Br (bromine) is an element with position number 35 in the periodic. All you need to do is work your way across the periodic table filling the orbitals as you go. Using the blocks in the periodic table. Use a chart such as the one below. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. Let’s use it to write the electron configuration of a neutral bromine atom, a bromine atom has 35 electrons. Electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas. ← electronic configurations of elements. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5.
from www.alamy.com
Bromine (br) is a chemical element. All you need to do is work your way across the periodic table filling the orbitals as you go. Let’s use it to write the electron configuration of a neutral bromine atom, a bromine atom has 35 electrons. ← electronic configurations of elements. Electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas. This can be shortened to [ar]4s23d104p5. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. The full version of this is: Using the blocks in the periodic table. Use a chart such as the one below.
Bromine (Br). Diagram of the nuclear composition and electron
Bromine Electron Configuration Long Form Bromine (br) is a chemical element. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. ← electronic configurations of elements. It evaporates readily and forms a colored gas. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. All you need to do is work your way across the periodic table filling the orbitals as you go. Let’s use it to write the electron configuration of a neutral bromine atom, a bromine atom has 35 electrons. Br (bromine) is an element with position number 35 in the periodic. Using the blocks in the periodic table. The full version of this is: Bromine (br) is a chemical element. This can be shortened to [ar]4s23d104p5. Use a chart such as the one below. Electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas. The atomic number of bromine is 35.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Bromine Electron Configuration Long Form All you need to do is work your way across the periodic table filling the orbitals as you go. The full version of this is: Let’s use it to write the electron configuration of a neutral bromine atom, a bromine atom has 35 electrons. ← electronic configurations of elements. Using the blocks in the periodic table. The electron configuration of. Bromine Electron Configuration Long Form.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electron Configuration Long Form Let’s use it to write the electron configuration of a neutral bromine atom, a bromine atom has 35 electrons. This can be shortened to [ar]4s23d104p5. Use a chart such as the one below. The atomic number of bromine is 35. The full version of this is: Electron configuration chart of all elements is mentioned in the table below.the shorthand electron. Bromine Electron Configuration Long Form.
From www.shutterstock.com
Symbol Electron Diagram Bromine Illustration Stock Vector (Royalty Free Bromine Electron Configuration Long Form This can be shortened to [ar]4s23d104p5. Bromine (br) is a chemical element. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas. ← electronic configurations of elements. The atomic number of bromine is 35. Br (bromine) is an element with position number 35 in the. Bromine Electron Configuration Long Form.
From www.youtube.com
Electron Configuration of Bromine, Br YouTube Bromine Electron Configuration Long Form ← electronic configurations of elements. This can be shortened to [ar]4s23d104p5. It evaporates readily and forms a colored gas. Electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas. The atomic number of bromine is 35. All you need to do is work your way across the periodic table filling the orbitals. Bromine Electron Configuration Long Form.
From www.youtube.com
Potassium Electron Configuration YouTube Bromine Electron Configuration Long Form The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The full version of this is: The atomic number of bromine is 35. Bromine (br) is a chemical element. All you need to do is work your way across the periodic table filling the orbitals as you go. Br (bromine) is an element with position number 35 in the periodic. Using the blocks. Bromine Electron Configuration Long Form.
From fyoekyjrv.blob.core.windows.net
Electron Configuration Of Bromine Long Form at Lowell Sadowski blog Bromine Electron Configuration Long Form It evaporates readily and forms a colored gas. Using the blocks in the periodic table. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The atomic number of bromine is 35. The full version of this is: Bromine (br) is a chemical element. Br (bromine) is an element with position number 35 in the periodic. Electron configuration chart of all elements is. Bromine Electron Configuration Long Form.
From fyoekyjrv.blob.core.windows.net
Electron Configuration Of Bromine Long Form at Lowell Sadowski blog Bromine Electron Configuration Long Form ← electronic configurations of elements. This can be shortened to [ar]4s23d104p5. All you need to do is work your way across the periodic table filling the orbitals as you go. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. Bromine (br) is. Bromine Electron Configuration Long Form.
From circuitwiringtrowel123.z13.web.core.windows.net
Box Diagram Of Electron Configuration Bromine Electron Configuration Long Form Let’s use it to write the electron configuration of a neutral bromine atom, a bromine atom has 35 electrons. Electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas. The full version of this is: Bromine (br) is a chemical element. The atomic number of bromine is 35. This can be shortened. Bromine Electron Configuration Long Form.
From fyoekyjrv.blob.core.windows.net
Electron Configuration Of Bromine Long Form at Lowell Sadowski blog Bromine Electron Configuration Long Form Electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas. Br (bromine) is an element with position number 35 in the periodic. This can be shortened to [ar]4s23d104p5. The full version of this is: The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Let’s use it to write the electron configuration of a neutral. Bromine Electron Configuration Long Form.
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube Bromine Electron Configuration Long Form It evaporates readily and forms a colored gas. This can be shortened to [ar]4s23d104p5. Use a chart such as the one below. ← electronic configurations of elements. The full version of this is: The atomic number of bromine is 35. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the. Bromine Electron Configuration Long Form.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Bromine Electron Configuration Long Form Br (bromine) is an element with position number 35 in the periodic. ← electronic configurations of elements. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. Let’s use it to write the electron configuration of. Bromine Electron Configuration Long Form.
From www.slideserve.com
PPT Entry Task October 19 th Friday PowerPoint Presentation, free Bromine Electron Configuration Long Form Br (bromine) is an element with position number 35 in the periodic. ← electronic configurations of elements. Using the blocks in the periodic table. Let’s use it to write the electron configuration of a neutral bromine atom, a bromine atom has 35 electrons. This can be shortened to [ar]4s23d104p5. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The atomic number of. Bromine Electron Configuration Long Form.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electron Configuration Long Form Electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas. The atomic number of bromine is 35. Use a chart such as the one below. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom.. Bromine Electron Configuration Long Form.
From favpng.com
Electron Configuration Bromine Chemical Element Electron Shell Bohr Bromine Electron Configuration Long Form The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The atomic number of bromine is 35. ← electronic configurations of elements. Electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas. This can be shortened to [ar]4s23d104p5. Let’s use it to write the electron configuration of a neutral bromine atom, a bromine atom has. Bromine Electron Configuration Long Form.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electron Configuration Long Form ← electronic configurations of elements. Br (bromine) is an element with position number 35 in the periodic. The full version of this is: The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. It evaporates readily and forms a colored gas. Use a. Bromine Electron Configuration Long Form.
From exogdjght.blob.core.windows.net
Magnesium Bromide Lewis Dot at Allen Matus blog Bromine Electron Configuration Long Form The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Br (bromine) is an element with position number 35 in the periodic. The full version of this is: Bromine (br) is a chemical element. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. It evaporates. Bromine Electron Configuration Long Form.
From www.vectorstock.com
Symbol and electron diagram for bromine Royalty Free Vector Bromine Electron Configuration Long Form Bromine (br) is a chemical element. Let’s use it to write the electron configuration of a neutral bromine atom, a bromine atom has 35 electrons. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Use a chart such as the one below. Electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas. Using the. Bromine Electron Configuration Long Form.
From periodictable.me
How Do We Find The Electron Configuration For Bromine Dynamic Bromine Electron Configuration Long Form Let’s use it to write the electron configuration of a neutral bromine atom, a bromine atom has 35 electrons. Bromine (br) is a chemical element. The full version of this is: Use a chart such as the one below. Electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas. The atomic number. Bromine Electron Configuration Long Form.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electron Configuration Long Form Using the blocks in the periodic table. This can be shortened to [ar]4s23d104p5. Use a chart such as the one below. It evaporates readily and forms a colored gas. The full version of this is: The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. All you need to do is work your way across the periodic table filling the orbitals as you. Bromine Electron Configuration Long Form.
From brainly.com
Which is the electron configuration for bromine? Bromine Electron Configuration Long Form Electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. This can be shortened to [ar]4s23d104p5. Br (bromine) is an element with position number 35. Bromine Electron Configuration Long Form.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Electron Configuration Long Form The atomic number of bromine is 35. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. All you need to do is work your way across the periodic table filling the orbitals as you go. Bromine (br) is a chemical element. It evaporates readily and forms a colored gas. ← electronic configurations of elements. Use a chart such as the one below.. Bromine Electron Configuration Long Form.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electron Configuration Long Form ← electronic configurations of elements. This can be shortened to [ar]4s23d104p5. The full version of this is: The atomic number of bromine is 35. All you need to do is work your way across the periodic table filling the orbitals as you go. Use a chart such as the one below. Br (bromine) is an element with position number 35. Bromine Electron Configuration Long Form.
From boisestate.pressbooks.pub
3.4 Electronic Structure of Atoms (Electron Configurations) General Bromine Electron Configuration Long Form ← electronic configurations of elements. Let’s use it to write the electron configuration of a neutral bromine atom, a bromine atom has 35 electrons. The atomic number of bromine is 35. Use a chart such as the one below. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise. Bromine Electron Configuration Long Form.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electron Configuration Long Form The atomic number of bromine is 35. The full version of this is: The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. Use a chart such as the one below. Br (bromine) is an element with position number 35 in the periodic.. Bromine Electron Configuration Long Form.
From ar.inspiredpencil.com
Electron Configuration Of Bromine Bromine Electron Configuration Long Form The full version of this is: It evaporates readily and forms a colored gas. Let’s use it to write the electron configuration of a neutral bromine atom, a bromine atom has 35 electrons. All you need to do is work your way across the periodic table filling the orbitals as you go. Use a chart such as the one below.. Bromine Electron Configuration Long Form.
From schematicpiscardixf.z4.web.core.windows.net
Orbital Diagram For Carbon Bromine Electron Configuration Long Form ← electronic configurations of elements. Br (bromine) is an element with position number 35 in the periodic. The full version of this is: Electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas. It evaporates readily and forms a colored gas. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. This can be shortened. Bromine Electron Configuration Long Form.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electron Configuration Long Form Let’s use it to write the electron configuration of a neutral bromine atom, a bromine atom has 35 electrons. Use a chart such as the one below. It evaporates readily and forms a colored gas. Electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas. The bromine electron configuration, denoted as 4s2. Bromine Electron Configuration Long Form.
From periodictable.me
How To Find A Electron Configuration For Silver (Ag) Bromine Electron Configuration Long Form The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Bromine (br) is a chemical element. This can be shortened to [ar]4s23d104p5. The full version of this is: The atomic number of bromine is 35. Let’s use it to write the electron configuration of a neutral bromine atom, a bromine atom has 35 electrons. ← electronic configurations of elements. Using the blocks in. Bromine Electron Configuration Long Form.
From lambdageeks.com
Bromine Electron Configuration 7 Easy Steps on How to Write Bromine Electron Configuration Long Form ← electronic configurations of elements. Use a chart such as the one below. The full version of this is: Bromine (br) is a chemical element. This can be shortened to [ar]4s23d104p5. Let’s use it to write the electron configuration of a neutral bromine atom, a bromine atom has 35 electrons. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The bromine electron. Bromine Electron Configuration Long Form.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Electron Configuration Long Form Bromine (br) is a chemical element. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. The full version of this is: The electron configuration of bromine is 1s22s22p63s23p64s23d104p5. Electron configuration chart of all elements is mentioned in the table below.the shorthand electron. Bromine Electron Configuration Long Form.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Electron Configuration Long Form All you need to do is work your way across the periodic table filling the orbitals as you go. Electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas. The atomic number of bromine is 35. It evaporates readily and forms a colored gas. Bromine (br) is a chemical element. The electron. Bromine Electron Configuration Long Form.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition and electron Bromine Electron Configuration Long Form The atomic number of bromine is 35. Let’s use it to write the electron configuration of a neutral bromine atom, a bromine atom has 35 electrons. Use a chart such as the one below. Br (bromine) is an element with position number 35 in the periodic. Electron configuration chart of all elements is mentioned in the table below.the shorthand electron. Bromine Electron Configuration Long Form.
From www.youtube.com
Super Trick Electronic configuration of Bromine Bromine electronic Bromine Electron Configuration Long Form The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. Use a chart such as the one below. The atomic number of bromine is 35. Br (bromine) is an element with position number 35 in the periodic. Bromine (br) is a chemical element.. Bromine Electron Configuration Long Form.
From www.youtube.com
Copy of Electron configuration, Orbital notation and Quantum Numbers Bromine Electron Configuration Long Form Let’s use it to write the electron configuration of a neutral bromine atom, a bromine atom has 35 electrons. Bromine (br) is a chemical element. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. Using the blocks in the periodic table. ←. Bromine Electron Configuration Long Form.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Electron Configuration Long Form The atomic number of bromine is 35. Let’s use it to write the electron configuration of a neutral bromine atom, a bromine atom has 35 electrons. Use a chart such as the one below. Bromine (br) is a chemical element. It evaporates readily and forms a colored gas. All you need to do is work your way across the periodic. Bromine Electron Configuration Long Form.