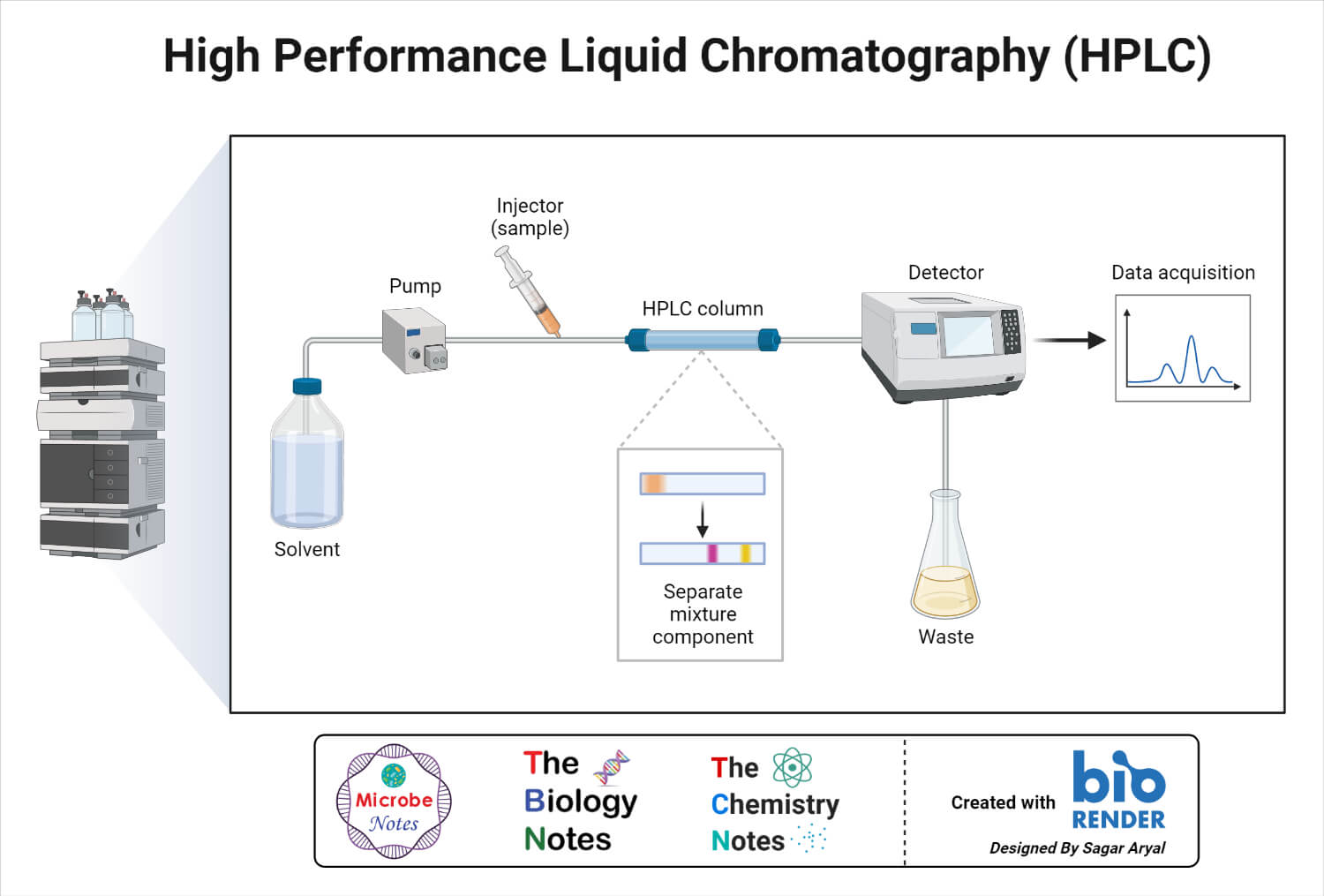
Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis . The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. One of the most critical factors in developing pharmaceutical drug substances and drug products today is ensuring that the hplc. Aiming to assist in the planning of validation methods, we discuss relevant approaches of various parameters in. The present study describes the risk based hplc method development and validation of ceftriaxone. A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an.
from www.gbu-presnenskij.ru
The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. The present study describes the risk based hplc method development and validation of ceftriaxone. One of the most critical factors in developing pharmaceutical drug substances and drug products today is ensuring that the hplc. Aiming to assist in the planning of validation methods, we discuss relevant approaches of various parameters in.
High Performance Liquid Chromatography (HPLC) Principle, 41 OFF
Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis One of the most critical factors in developing pharmaceutical drug substances and drug products today is ensuring that the hplc. Aiming to assist in the planning of validation methods, we discuss relevant approaches of various parameters in. One of the most critical factors in developing pharmaceutical drug substances and drug products today is ensuring that the hplc. The present study describes the risk based hplc method development and validation of ceftriaxone. The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an.
From www.researchgate.net
(PDF) Development and Validation of High Performance Liquid Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. Aiming to assist in the planning of validation methods, we discuss relevant approaches of various parameters in. The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. The present study describes the risk based hplc method development and validation. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.researchgate.net
(PDF) Analytical Validation of Quantitative HighPerformance Liquid Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis One of the most critical factors in developing pharmaceutical drug substances and drug products today is ensuring that the hplc. The present study describes the risk based hplc method development and validation of ceftriaxone. A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. The present manuscript, describes several strategies and applications of. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.academia.edu
(PDF) Development and validation of high performance liquid Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis Aiming to assist in the planning of validation methods, we discuss relevant approaches of various parameters in. The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. The present study describes the risk based hplc method development and validation. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.youtube.com
HPLC High Performance Liquid Chromatography Application of HPLC Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis One of the most critical factors in developing pharmaceutical drug substances and drug products today is ensuring that the hplc. A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. The present study describes the risk based hplc method development and validation of ceftriaxone. The present manuscript, describes several strategies and applications of. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.vrogue.co
Hplc High Performance Liquid Chromatography Introduct vrogue.co Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis The present study describes the risk based hplc method development and validation of ceftriaxone. A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. One of the most critical factors in developing pharmaceutical drug substances and drug products today is ensuring that the hplc. The present manuscript, describes several strategies and applications of. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.semanticscholar.org
Figure 2 from Development and validation of a high performance liquid Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. Aiming to assist in the planning of validation methods, we discuss relevant approaches of various parameters in. One of the most critical factors in developing pharmaceutical drug substances and. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.researchgate.net
(PDF) High Performance Liquid Chromatographic Method Development and Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis One of the most critical factors in developing pharmaceutical drug substances and drug products today is ensuring that the hplc. A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. The present study describes the risk based hplc method development and validation of ceftriaxone. Aiming to assist in the planning of validation methods,. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.semanticscholar.org
Figure 9 from DEVELOPMENT AND VALIDATION OF STABILITY INDICATING Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. One of the most critical factors in developing pharmaceutical drug substances and drug products today is ensuring that the hplc. A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. Aiming to assist in the planning of validation methods,. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.researchgate.net
(PDF) Validation of high performance liquid chromatographic method for Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. One of the most critical factors in developing pharmaceutical drug substances and drug products today is ensuring that the hplc. Aiming to assist in the planning of validation methods,. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.researchgate.net
(PDF) Development and Validation of High Performance Liquid Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis Aiming to assist in the planning of validation methods, we discuss relevant approaches of various parameters in. A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. One of the most critical factors in developing pharmaceutical drug substances and. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.researchgate.net
(PDF) Development and Validation of Reverse Phase High Performance Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. The present study describes the risk based hplc method development and validation of ceftriaxone. One of the most critical factors in developing pharmaceutical drug substances and drug products today. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.trivitron.com
Trivitron HighPerformance Liquid Chromatography Systems for Accurate Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. The present study describes the risk based hplc method development and validation of ceftriaxone. One of the most critical factors in developing pharmaceutical drug substances and drug products today is ensuring that the hplc. Aiming to assist in the planning of validation methods,. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.scribd.com
Development and Validation of HPLC Chromatography Method For Nystatin Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. Aiming to assist in the planning of validation methods, we discuss relevant approaches of various parameters in. The present study describes the risk based hplc method development and validation of ceftriaxone. A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.researchgate.net
(PDF) VALIDATION OF HIGH PERFORMANCE LIQUID CHROMATOGRAPHY (HPLC Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. One of the most critical factors in developing pharmaceutical drug substances and drug products today is ensuring that the hplc. The present study describes the risk based hplc method. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.researchgate.net
(PDF) Development and validation of a new highperformance liquid Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis The present study describes the risk based hplc method development and validation of ceftriaxone. A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. Aiming to assist in the planning of validation methods, we discuss relevant approaches of various parameters in. The present manuscript, describes several strategies and applications of chemometry, specially focusing. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.researchgate.net
(PDF) StabilityIndicating ReversePhase HighPerformance Liquid Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. The present study describes the risk based hplc method development and validation of ceftriaxone. A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. One of the most critical factors in developing pharmaceutical drug substances and drug products today. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.researchgate.net
(PDF) REVERSE PHASE HIGH PERFORMANCE LIQUID CHROMATOGRAPHIC METHOD Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis The present study describes the risk based hplc method development and validation of ceftriaxone. A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. One of the most critical factors in developing pharmaceutical drug substances and drug products today is ensuring that the hplc. The present manuscript, describes several strategies and applications of. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.priyamstudycentre.com
High Performance Liquid Chromatography (HPLC) Principle Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis One of the most critical factors in developing pharmaceutical drug substances and drug products today is ensuring that the hplc. A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. Aiming to assist in the planning of validation methods,. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.researchgate.net
(PDF) Development and Validation of a StabilityIndicating High Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis One of the most critical factors in developing pharmaceutical drug substances and drug products today is ensuring that the hplc. The present study describes the risk based hplc method development and validation of ceftriaxone. Aiming to assist in the planning of validation methods, we discuss relevant approaches of various parameters in. A reverse phase ultra performance liquid chromatographic method was. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.researchgate.net
(PDF) "VALIDATION OF STABILITY INDICATING HIGH PERFORMANCE LIQUID Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis The present study describes the risk based hplc method development and validation of ceftriaxone. The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. One of the most critical factors in developing pharmaceutical drug substances and drug products today is ensuring that the hplc. Aiming to assist in the planning of validation methods, we discuss relevant. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.researchgate.net
(PDF) Development and Validation of a Simple, Rapid and Stability Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. Aiming to assist in the planning of validation methods, we discuss relevant approaches of various parameters in. The present study describes the risk based hplc method development and validation. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.researchgate.net
(PDF) DEVELOPMENT AND VALIDATION OF STABILITYINDICATING REVERSE PHASE Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. Aiming to assist in the planning of validation methods, we discuss relevant approaches of various parameters in. The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. The present study describes the risk based hplc method development and validation. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.researchgate.net
(PDF) TO VALIDATE STABILITY INDICATING HIGH PERFORMANCE LIQUID Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. One of the most critical factors in developing pharmaceutical drug substances and drug products today is ensuring that the hplc. The present study describes the risk based hplc method development and validation of ceftriaxone. The present manuscript, describes several strategies and applications of. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.researchgate.net
Highperformance liquid chromatography (HPLC) analysis showing the Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis The present study describes the risk based hplc method development and validation of ceftriaxone. A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. One of the most critical factors in developing pharmaceutical drug substances and drug products today is ensuring that the hplc. The present manuscript, describes several strategies and applications of. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.researchgate.net
(PDF) HighPerformance Liquid Chromatographic Method for Analysis of Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. One of the most critical factors in developing pharmaceutical drug substances and drug products today is ensuring that the hplc. The present study describes the risk based hplc method. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.shimadzu.com
What is HPLC (High Performance Liquid Chromatography) ? SHIMADZU Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. One of the most critical factors in developing pharmaceutical drug substances and drug products today is ensuring that the hplc. The present study describes the risk based hplc method development and validation of ceftriaxone. Aiming to assist in the planning of validation methods, we discuss relevant. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.gbu-presnenskij.ru
High Performance Liquid Chromatography (HPLC) Principle, 41 OFF Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. One of the most critical factors in developing pharmaceutical drug substances and drug products today is ensuring that the hplc. The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. Aiming to assist in the planning of validation methods,. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.researchgate.net
(PDF) Validation of a highperformance liquid chromatographic Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. One of the most critical factors in developing pharmaceutical drug substances and drug products today is ensuring that the hplc. The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. The present study describes the risk based hplc method. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.researchgate.net
(PDF) Development and Validation of a Simple and Sensitive Reverse Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis One of the most critical factors in developing pharmaceutical drug substances and drug products today is ensuring that the hplc. The present study describes the risk based hplc method development and validation of ceftriaxone. The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. A reverse phase ultra performance liquid chromatographic method was developed and validated. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.pharmacy.cuhk.edu.hk
lab2High Performance Liquid Chromatographic System (HPLC) (ID1.3 Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. Aiming to assist in the planning of validation methods, we discuss relevant approaches of various parameters in. The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. The present study describes the risk based hplc method development and validation. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.researchgate.net
(PDF) Validation of Reverse Phase Highperformance Liquid Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis The present study describes the risk based hplc method development and validation of ceftriaxone. Aiming to assist in the planning of validation methods, we discuss relevant approaches of various parameters in. The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.researchgate.net
(PDF) Development and Validation of a HighPerformance Liquid Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis One of the most critical factors in developing pharmaceutical drug substances and drug products today is ensuring that the hplc. A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. Aiming to assist in the planning of validation methods, we discuss relevant approaches of various parameters in. The present study describes the risk. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.academia.edu
(PDF) Validation of a highperformance liquid chromatographic method Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis The present study describes the risk based hplc method development and validation of ceftriaxone. The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. One of the most critical factors in developing pharmaceutical drug substances and drug products today. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.semanticscholar.org
Table 1 from DEVELOPMENT AND VALIDATION OF REVERSEDPHASE HIGH Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. One of the most critical factors in developing pharmaceutical drug substances and drug products today is ensuring that the hplc. Aiming to assist in the planning of validation methods,. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.
From www.semanticscholar.org
Figure 2 from Development and Validation of ReversedPhase High Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis Aiming to assist in the planning of validation methods, we discuss relevant approaches of various parameters in. The present manuscript, describes several strategies and applications of chemometry, specially focusing on those. A reverse phase ultra performance liquid chromatographic method was developed and validated for the quantitation of an. The present study describes the risk based hplc method development and validation. Validation Of High-Performance Liquid Chromatography Methods For Pharmaceutical Analysis.