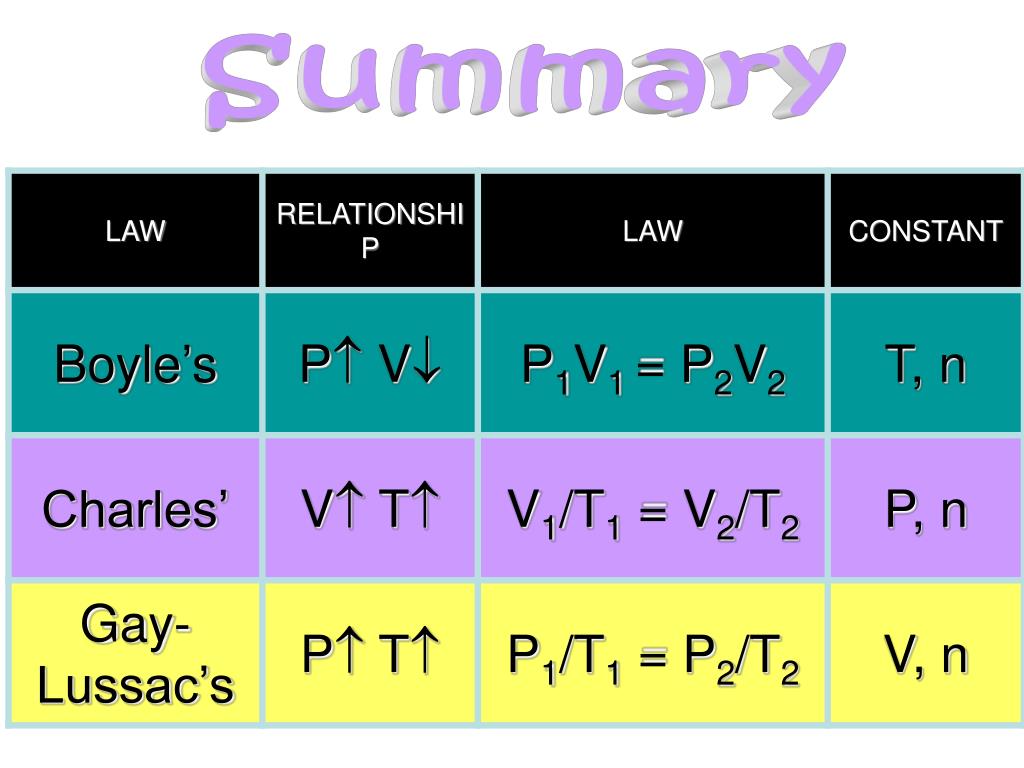
Air Pressure Laws . This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. When you inflate a balloon, the air molecules inside the balloon get packed more closely together than air molecules outside the. I.e., in equation form, pv = k, a constant. Learn about atmospheric pressure and its units and methods of measurement description of pressure and its measurement. Learn what is meant by the term gas laws. In other words, the pressure and volume of a gas are inversely proportional to each other as long as the temperature and the quantity of gas are kept constant. Air pressure depends on temperature and density. Learn and apply boyle’s law. Boyle's law predicts that at two atmospheres pressure (33 fsw) the volume of air in the container will decrease by one half and the density of air will double. Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume occupied by it.
from www.slideserve.com
Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. Learn about atmospheric pressure and its units and methods of measurement description of pressure and its measurement. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Boyle's law predicts that at two atmospheres pressure (33 fsw) the volume of air in the container will decrease by one half and the density of air will double. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; Learn what is meant by the term gas laws. Air pressure depends on temperature and density. When you inflate a balloon, the air molecules inside the balloon get packed more closely together than air molecules outside the. Learn and apply boyle’s law. Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume occupied by it.
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint
Air Pressure Laws Learn about atmospheric pressure and its units and methods of measurement description of pressure and its measurement. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; I.e., in equation form, pv = k, a constant. Learn and apply boyle’s law. Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. Learn what is meant by the term gas laws. When you inflate a balloon, the air molecules inside the balloon get packed more closely together than air molecules outside the. Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume occupied by it. In other words, the pressure and volume of a gas are inversely proportional to each other as long as the temperature and the quantity of gas are kept constant. Air pressure depends on temperature and density. Learn about atmospheric pressure and its units and methods of measurement description of pressure and its measurement. Boyle's law predicts that at two atmospheres pressure (33 fsw) the volume of air in the container will decrease by one half and the density of air will double. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows:
From www.dreamstime.com
Ideal Gas Law. Boyles Law Pressure Volume Relationship in Gases Stock Air Pressure Laws Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume occupied by it. In other words, the pressure and volume of a gas are inversely. Air Pressure Laws.
From studylib.net
Pressure law Air Pressure Laws This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume occupied by it. This empirical relation, formulated by the physicist. Air Pressure Laws.
From atomstalk.com
The Gas Laws AtomsTalk Air Pressure Laws Learn about atmospheric pressure and its units and methods of measurement description of pressure and its measurement. Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume occupied by it. Air pressure depends on temperature and density. This relationship between. Air Pressure Laws.
From www.askmattrab.com
Pressure Liquid Pressure, Pascal's Law and Class Ten Science Air Pressure Laws This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; Learn about atmospheric pressure and its units and methods of measurement description of pressure and its measurement. I.e., in equation form, pv = k, a constant. When you inflate. Air Pressure Laws.
From drcalef.com
The Combined Gas Law Pressure, Volume, and Temperature Air Pressure Laws Learn and apply boyle’s law. Boyle's law predicts that at two atmospheres pressure (33 fsw) the volume of air in the container will decrease by one half and the density of air will double. Learn about atmospheric pressure and its units and methods of measurement description of pressure and its measurement. Learn what is meant by the term gas laws.. Air Pressure Laws.
From mmerevise.co.uk
The Ideal Gas Equation MME Air Pressure Laws Learn and apply boyle’s law. I.e., in equation form, pv = k, a constant. Learn what is meant by the term gas laws. In other words, the pressure and volume of a gas are inversely proportional to each other as long as the temperature and the quantity of gas are kept constant. Air pressure depends on temperature and density. Boyle’s. Air Pressure Laws.
From www.savemyexams.com
5.3.5 The Pressure Law Edexcel IGCSE Physics Revision Notes 2019 Air Pressure Laws Learn what is meant by the term gas laws. Learn and apply boyle’s law. Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: When you inflate a balloon, the air molecules inside the balloon. Air Pressure Laws.
From www.nesscopressure.com.au
What is air pressure? Nessco Pressure Systems Air Pressure Laws This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; When you inflate a balloon, the air molecules inside the balloon get packed more closely together than air molecules outside the. Learn and apply boyle’s law. Boyle's law predicts. Air Pressure Laws.
From stock.adobe.com
Gay Lussac Law Infographic Diagram example of pressure cooker Air Pressure Laws In other words, the pressure and volume of a gas are inversely proportional to each other as long as the temperature and the quantity of gas are kept constant. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature;. Air Pressure Laws.
From www.sciencefacts.net
Pascal’s Law Statement, Formula, Examples, & Application Air Pressure Laws Air pressure depends on temperature and density. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; Learn about atmospheric pressure and its units and methods of measurement description of pressure and its measurement. Learn and apply boyle’s law.. Air Pressure Laws.
From brainly.com
Charles's law is an experimental gas law that shows the relationship Air Pressure Laws Learn what is meant by the term gas laws. Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: In other words, the pressure and volume of a gas are inversely proportional to each other. Air Pressure Laws.
From www.inspiritvr.com
GayLussac’s Law of Ideal Gasses Study Guide Inspirit Air Pressure Laws Boyle's law predicts that at two atmospheres pressure (33 fsw) the volume of air in the container will decrease by one half and the density of air will double. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature;. Air Pressure Laws.
From www.youtube.com
Pressure Law Heat Physics YouTube Air Pressure Laws Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume occupied by it. Air pressure depends on temperature and density. Boyle's law predicts that at two atmospheres pressure (33 fsw) the volume of air in the container will decrease by. Air Pressure Laws.
From www.expii.com
Combined Gas Law — Overview & Calculations Expii Air Pressure Laws In other words, the pressure and volume of a gas are inversely proportional to each other as long as the temperature and the quantity of gas are kept constant. Air pressure depends on temperature and density. Learn what is meant by the term gas laws. I.e., in equation form, pv = k, a constant. When you inflate a balloon, the. Air Pressure Laws.
From chart-studio.plotly.com
Boyle's Law PressureVolume Relationship in Gases scatter chart made Air Pressure Laws Learn about atmospheric pressure and its units and methods of measurement description of pressure and its measurement. Boyle's law predicts that at two atmospheres pressure (33 fsw) the volume of air in the container will decrease by one half and the density of air will double. Boyle’s law, a relation concerning the compression and expansion of a gas at constant. Air Pressure Laws.
From blog.fluidflowinfo.com
Fan Laws and Fan Performance Fluid Flow Engineering FluidFlow Air Pressure Laws Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: I.e., in equation form, pv = k, a constant. Boyle’s law is a gas law which states that the pressure exerted by a gas (of. Air Pressure Laws.
From www.tessshebaylo.com
Write The Mathematical Equation For Charles Law And Explain Symbols Air Pressure Laws Learn what is meant by the term gas laws. Boyle's law predicts that at two atmospheres pressure (33 fsw) the volume of air in the container will decrease by one half and the density of air will double. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas. Air Pressure Laws.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint Air Pressure Laws Air pressure depends on temperature and density. I.e., in equation form, pv = k, a constant. Boyle's law predicts that at two atmospheres pressure (33 fsw) the volume of air in the container will decrease by one half and the density of air will double. In other words, the pressure and volume of a gas are inversely proportional to each. Air Pressure Laws.
From med.libretexts.org
2.3 Gaseous Exchange Mechanism Medicine LibreTexts Air Pressure Laws Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Air pressure depends on temperature and density. Boyle's law predicts that at two atmospheres pressure (33 fsw) the volume of air in the container will. Air Pressure Laws.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint Air Pressure Laws This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Learn what is meant by the term gas laws. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature;. Air Pressure Laws.
From www.slideserve.com
PPT Dalton’s Law of Partial Pressures PowerPoint Presentation, free Air Pressure Laws I.e., in equation form, pv = k, a constant. When you inflate a balloon, the air molecules inside the balloon get packed more closely together than air molecules outside the. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant. Air Pressure Laws.
From www.slideserve.com
PPT Dalton’s Law The total pressure of a mixture of gases equals the Air Pressure Laws Boyle's law predicts that at two atmospheres pressure (33 fsw) the volume of air in the container will decrease by one half and the density of air will double. I.e., in equation form, pv = k, a constant. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas. Air Pressure Laws.
From general.chemistrysteps.com
Boyle’s Law Chemistry Steps Air Pressure Laws Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume occupied by it. Learn about atmospheric pressure and its units and methods of measurement description of pressure and its measurement. I.e., in equation form, pv = k, a constant. Air. Air Pressure Laws.
From groups.google.com
Tell us in your own words what it is you think you see here Air Pressure Laws I.e., in equation form, pv = k, a constant. In other words, the pressure and volume of a gas are inversely proportional to each other as long as the temperature and the quantity of gas are kept constant. When you inflate a balloon, the air molecules inside the balloon get packed more closely together than air molecules outside the. Learn. Air Pressure Laws.
From www.upsite.com
[VIDEO] How Fan Affinity Laws Impact Fan Energy Savings Air Pressure Laws Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume occupied by it. Boyle's law predicts that at two atmospheres pressure (33 fsw) the volume. Air Pressure Laws.
From sciencenotes.org
Charles's Law Definition, Formula, Examples Air Pressure Laws Air pressure depends on temperature and density. When you inflate a balloon, the air molecules inside the balloon get packed more closely together than air molecules outside the. Boyle's law predicts that at two atmospheres pressure (33 fsw) the volume of air in the container will decrease by one half and the density of air will double. This relationship between. Air Pressure Laws.
From www.youtube.com
Pascal's Law, Hydrostatic Pressure and Barometers to Measure Air Pressure Laws Learn about atmospheric pressure and its units and methods of measurement description of pressure and its measurement. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; Boyle’s law is a gas law which states that the pressure exerted. Air Pressure Laws.
From fr.slideshare.net
Physics form 4 chapter 4 slides Air Pressure Laws I.e., in equation form, pv = k, a constant. Learn about atmospheric pressure and its units and methods of measurement description of pressure and its measurement. In other words, the pressure and volume of a gas are inversely proportional to each other as long as the temperature and the quantity of gas are kept constant. Learn what is meant by. Air Pressure Laws.
From physicscalculations.com
What is Pressure Law? Air Pressure Laws I.e., in equation form, pv = k, a constant. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. In other words, the pressure. Air Pressure Laws.
From sciencenotes.org
Ideal Gas Law Formula and Examples Air Pressure Laws Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume occupied by it. In other words, the pressure and volume of a gas are inversely proportional to each other as long as the temperature and the quantity of gas are. Air Pressure Laws.
From www.britannica.com
Boyle’s law Definition, Equation, & Facts Britannica Air Pressure Laws This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Boyle's law predicts that at two atmospheres pressure (33 fsw) the volume of air in the container will decrease by one half and the density of air will double. Learn what is meant by the term gas laws. Boyle’s law,. Air Pressure Laws.
From www.britannica.com
Ideal gas law Definition, Formula, & Facts Britannica Air Pressure Laws This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: I.e., in equation form, pv = k, a constant. Boyle’s law, a relation concerning the compression and expansion of a gas at constant temperature. Learn what is meant by the term gas laws. When you inflate a balloon, the air. Air Pressure Laws.
From www.chevyhardcore.com
Air Density The Pillar of Performance Revealed With Gale Banks Air Pressure Laws In other words, the pressure and volume of a gas are inversely proportional to each other as long as the temperature and the quantity of gas are kept constant. This relationship between pressure and volume is known as boyle’s law, after its discoverer, and can be stated as follows: Learn about atmospheric pressure and its units and methods of measurement. Air Pressure Laws.
From studiousguy.com
8 Boyle’s Law Examples in Real Life StudiousGuy Air Pressure Laws In other words, the pressure and volume of a gas are inversely proportional to each other as long as the temperature and the quantity of gas are kept constant. This empirical relation, formulated by the physicist robert boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature;. Air Pressure Laws.
From www.studypool.com
SOLUTION Gas laws key worksheet with answer Studypool Air Pressure Laws Learn about atmospheric pressure and its units and methods of measurement description of pressure and its measurement. Boyle’s law is a gas law which states that the pressure exerted by a gas (of a given mass, kept at a constant temperature) is inversely proportional to the volume occupied by it. When you inflate a balloon, the air molecules inside the. Air Pressure Laws.