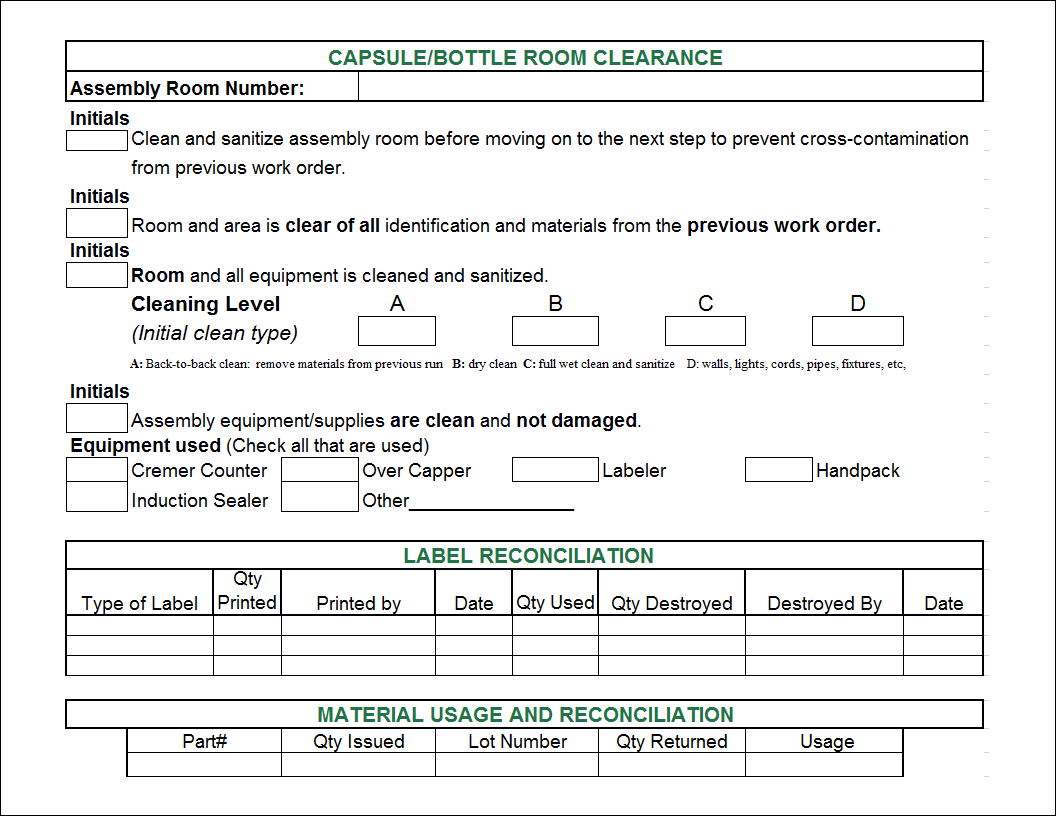
Master Batch Record In Pharmaceutical Industry . The batch manufacturing record (bmr) is the compiled document created during the manufacturing process for each specific batch, based on the master batch record (mbr). Learn how to prepare, review, approve, issue, maintain, and archive controlled master batch record (mbr) for pharmaceutical. Master batch record (mbr) ensures consistency and accuracy in manufacturing processes. A master batch record (mbr), or master production record, is a comprehensive document that serves as the manufacturing. It outlines every step of the manufacturing. A master batch record (mbr) contains the instructions, recipe or formula, and specific manufacturing process for a particular product. It ensures that each batch is produced in compliance Learn about the aims, objectives, key components,. Mbr is a comprehensive document that outlines the instructions for manufacturing a specific product batch. The master batch record (mbr) is the foundation of all batch documentation. How does mbr help in pharma?
from old.sermitsiaq.ag
The batch manufacturing record (bmr) is the compiled document created during the manufacturing process for each specific batch, based on the master batch record (mbr). It ensures that each batch is produced in compliance It outlines every step of the manufacturing. Learn about the aims, objectives, key components,. Learn how to prepare, review, approve, issue, maintain, and archive controlled master batch record (mbr) for pharmaceutical. Master batch record (mbr) ensures consistency and accuracy in manufacturing processes. A master batch record (mbr), or master production record, is a comprehensive document that serves as the manufacturing. The master batch record (mbr) is the foundation of all batch documentation. Mbr is a comprehensive document that outlines the instructions for manufacturing a specific product batch. A master batch record (mbr) contains the instructions, recipe or formula, and specific manufacturing process for a particular product.
Master Batch Record Template
Master Batch Record In Pharmaceutical Industry Learn how to prepare, review, approve, issue, maintain, and archive controlled master batch record (mbr) for pharmaceutical. How does mbr help in pharma? Master batch record (mbr) ensures consistency and accuracy in manufacturing processes. The batch manufacturing record (bmr) is the compiled document created during the manufacturing process for each specific batch, based on the master batch record (mbr). It outlines every step of the manufacturing. Learn how to prepare, review, approve, issue, maintain, and archive controlled master batch record (mbr) for pharmaceutical. Learn about the aims, objectives, key components,. A master batch record (mbr), or master production record, is a comprehensive document that serves as the manufacturing. It ensures that each batch is produced in compliance The master batch record (mbr) is the foundation of all batch documentation. Mbr is a comprehensive document that outlines the instructions for manufacturing a specific product batch. A master batch record (mbr) contains the instructions, recipe or formula, and specific manufacturing process for a particular product.
From www.slideshare.net
Electronic batch record management in pharmaceutical industry Master Batch Record In Pharmaceutical Industry The master batch record (mbr) is the foundation of all batch documentation. Master batch record (mbr) ensures consistency and accuracy in manufacturing processes. It outlines every step of the manufacturing. Learn how to prepare, review, approve, issue, maintain, and archive controlled master batch record (mbr) for pharmaceutical. How does mbr help in pharma? Learn about the aims, objectives, key components,.. Master Batch Record In Pharmaceutical Industry.
From www.poms.com
Electronic Batch Records in 2024 for Advanced Pharma & Biotech Master Batch Record In Pharmaceutical Industry Learn how to prepare, review, approve, issue, maintain, and archive controlled master batch record (mbr) for pharmaceutical. It outlines every step of the manufacturing. It ensures that each batch is produced in compliance Master batch record (mbr) ensures consistency and accuracy in manufacturing processes. How does mbr help in pharma? Mbr is a comprehensive document that outlines the instructions for. Master Batch Record In Pharmaceutical Industry.
From instantgmp.com
InstantGMP™ PRO Electronic Batch Record Management Software Master Batch Record In Pharmaceutical Industry The batch manufacturing record (bmr) is the compiled document created during the manufacturing process for each specific batch, based on the master batch record (mbr). Mbr is a comprehensive document that outlines the instructions for manufacturing a specific product batch. Master batch record (mbr) ensures consistency and accuracy in manufacturing processes. Learn how to prepare, review, approve, issue, maintain, and. Master Batch Record In Pharmaceutical Industry.
From www.cphi-online.com
Electronic Batch Manufacturing Record (eBMR) Samvardhana Motherson Master Batch Record In Pharmaceutical Industry Master batch record (mbr) ensures consistency and accuracy in manufacturing processes. Learn how to prepare, review, approve, issue, maintain, and archive controlled master batch record (mbr) for pharmaceutical. It outlines every step of the manufacturing. It ensures that each batch is produced in compliance Learn about the aims, objectives, key components,. A master batch record (mbr) contains the instructions, recipe. Master Batch Record In Pharmaceutical Industry.
From www.slideshare.net
Documentation in Pharmaceutical Industry PPT Master Batch Record In Pharmaceutical Industry How does mbr help in pharma? A master batch record (mbr) contains the instructions, recipe or formula, and specific manufacturing process for a particular product. A master batch record (mbr), or master production record, is a comprehensive document that serves as the manufacturing. It outlines every step of the manufacturing. The batch manufacturing record (bmr) is the compiled document created. Master Batch Record In Pharmaceutical Industry.
From sgsystemsglobal.com
Electronic Batch Record (EBR) Batch Manufacturing Record (MBR) Master Batch Record In Pharmaceutical Industry Learn about the aims, objectives, key components,. How does mbr help in pharma? A master batch record (mbr), or master production record, is a comprehensive document that serves as the manufacturing. The batch manufacturing record (bmr) is the compiled document created during the manufacturing process for each specific batch, based on the master batch record (mbr). The master batch record. Master Batch Record In Pharmaceutical Industry.
From www.slideteam.net
Pharmaceutical Quality Control And Assurance Functions Master Batch Record In Pharmaceutical Industry How does mbr help in pharma? Mbr is a comprehensive document that outlines the instructions for manufacturing a specific product batch. It ensures that each batch is produced in compliance A master batch record (mbr), or master production record, is a comprehensive document that serves as the manufacturing. Learn how to prepare, review, approve, issue, maintain, and archive controlled master. Master Batch Record In Pharmaceutical Industry.
From www.gmpdocs.com
Batch Record Review Checklist Template/Example Master Batch Record In Pharmaceutical Industry The batch manufacturing record (bmr) is the compiled document created during the manufacturing process for each specific batch, based on the master batch record (mbr). A master batch record (mbr), or master production record, is a comprehensive document that serves as the manufacturing. A master batch record (mbr) contains the instructions, recipe or formula, and specific manufacturing process for a. Master Batch Record In Pharmaceutical Industry.
From www.slideshare.net
BMR (Batch Manufacturing Record) Master Batch Record In Pharmaceutical Industry A master batch record (mbr) contains the instructions, recipe or formula, and specific manufacturing process for a particular product. A master batch record (mbr), or master production record, is a comprehensive document that serves as the manufacturing. It ensures that each batch is produced in compliance The master batch record (mbr) is the foundation of all batch documentation. Learn about. Master Batch Record In Pharmaceutical Industry.
From www.datacor.com
2024 Batch Manufacturing Record Definition, Template & Examples Master Batch Record In Pharmaceutical Industry It outlines every step of the manufacturing. How does mbr help in pharma? A master batch record (mbr) contains the instructions, recipe or formula, and specific manufacturing process for a particular product. Mbr is a comprehensive document that outlines the instructions for manufacturing a specific product batch. The master batch record (mbr) is the foundation of all batch documentation. A. Master Batch Record In Pharmaceutical Industry.
From es.scribd.com
ASEAN TMHS GMP Training Chapter 5 Annex 5 Sample Batch Manufacturing Master Batch Record In Pharmaceutical Industry A master batch record (mbr), or master production record, is a comprehensive document that serves as the manufacturing. The batch manufacturing record (bmr) is the compiled document created during the manufacturing process for each specific batch, based on the master batch record (mbr). The master batch record (mbr) is the foundation of all batch documentation. Master batch record (mbr) ensures. Master Batch Record In Pharmaceutical Industry.
From www.youtube.com
Batch Formula Record, Master Formula Record, Standard Operating Master Batch Record In Pharmaceutical Industry It outlines every step of the manufacturing. Learn how to prepare, review, approve, issue, maintain, and archive controlled master batch record (mbr) for pharmaceutical. Mbr is a comprehensive document that outlines the instructions for manufacturing a specific product batch. A master batch record (mbr) contains the instructions, recipe or formula, and specific manufacturing process for a particular product. The master. Master Batch Record In Pharmaceutical Industry.
From old.sermitsiaq.ag
Master Manufacturing Record Template Master Batch Record In Pharmaceutical Industry It ensures that each batch is produced in compliance Learn about the aims, objectives, key components,. Mbr is a comprehensive document that outlines the instructions for manufacturing a specific product batch. The master batch record (mbr) is the foundation of all batch documentation. How does mbr help in pharma? It outlines every step of the manufacturing. A master batch record. Master Batch Record In Pharmaceutical Industry.
From pharmaceuticalsindex.com
Different Types of Batch Records in Pharmaceutical Industry Master Batch Record In Pharmaceutical Industry It ensures that each batch is produced in compliance It outlines every step of the manufacturing. Learn about the aims, objectives, key components,. How does mbr help in pharma? Learn how to prepare, review, approve, issue, maintain, and archive controlled master batch record (mbr) for pharmaceutical. Mbr is a comprehensive document that outlines the instructions for manufacturing a specific product. Master Batch Record In Pharmaceutical Industry.
From datamyte.com
Master Batch Record Your Essential Guide DataMyte Master Batch Record In Pharmaceutical Industry Learn how to prepare, review, approve, issue, maintain, and archive controlled master batch record (mbr) for pharmaceutical. It outlines every step of the manufacturing. Mbr is a comprehensive document that outlines the instructions for manufacturing a specific product batch. A master batch record (mbr), or master production record, is a comprehensive document that serves as the manufacturing. How does mbr. Master Batch Record In Pharmaceutical Industry.
From scicord.com
Batch Records increase efficiency and compliance SciCord Master Batch Record In Pharmaceutical Industry The batch manufacturing record (bmr) is the compiled document created during the manufacturing process for each specific batch, based on the master batch record (mbr). It outlines every step of the manufacturing. Learn about the aims, objectives, key components,. Learn how to prepare, review, approve, issue, maintain, and archive controlled master batch record (mbr) for pharmaceutical. Mbr is a comprehensive. Master Batch Record In Pharmaceutical Industry.
From www.access-programmers.co.uk
Designing a batch production record database Access World Forums Master Batch Record In Pharmaceutical Industry A master batch record (mbr), or master production record, is a comprehensive document that serves as the manufacturing. A master batch record (mbr) contains the instructions, recipe or formula, and specific manufacturing process for a particular product. The master batch record (mbr) is the foundation of all batch documentation. Master batch record (mbr) ensures consistency and accuracy in manufacturing processes.. Master Batch Record In Pharmaceutical Industry.
From www.scribd.com
Check List of Batch Record Production And Manufacturing Industrial Master Batch Record In Pharmaceutical Industry How does mbr help in pharma? Master batch record (mbr) ensures consistency and accuracy in manufacturing processes. The batch manufacturing record (bmr) is the compiled document created during the manufacturing process for each specific batch, based on the master batch record (mbr). Learn how to prepare, review, approve, issue, maintain, and archive controlled master batch record (mbr) for pharmaceutical. Mbr. Master Batch Record In Pharmaceutical Industry.
From www.youtube.com
Batch manufacturing recordBMR official documentation in Master Batch Record In Pharmaceutical Industry How does mbr help in pharma? Learn about the aims, objectives, key components,. It ensures that each batch is produced in compliance Master batch record (mbr) ensures consistency and accuracy in manufacturing processes. Learn how to prepare, review, approve, issue, maintain, and archive controlled master batch record (mbr) for pharmaceutical. Mbr is a comprehensive document that outlines the instructions for. Master Batch Record In Pharmaceutical Industry.
From www.wholesalesuppliesplus.com
Formula & Batch Records Wholesale Supplies Plus Master Batch Record In Pharmaceutical Industry Master batch record (mbr) ensures consistency and accuracy in manufacturing processes. Mbr is a comprehensive document that outlines the instructions for manufacturing a specific product batch. It outlines every step of the manufacturing. A master batch record (mbr), or master production record, is a comprehensive document that serves as the manufacturing. The master batch record (mbr) is the foundation of. Master Batch Record In Pharmaceutical Industry.
From mavink.com
Batch Production Record Templates Master Batch Record In Pharmaceutical Industry Learn about the aims, objectives, key components,. How does mbr help in pharma? The batch manufacturing record (bmr) is the compiled document created during the manufacturing process for each specific batch, based on the master batch record (mbr). Master batch record (mbr) ensures consistency and accuracy in manufacturing processes. A master batch record (mbr) contains the instructions, recipe or formula,. Master Batch Record In Pharmaceutical Industry.
From www.slideshare.net
Master batch record,batch production record ,Quality Audit Type and p… Master Batch Record In Pharmaceutical Industry A master batch record (mbr), or master production record, is a comprehensive document that serves as the manufacturing. Learn about the aims, objectives, key components,. It outlines every step of the manufacturing. The master batch record (mbr) is the foundation of all batch documentation. Mbr is a comprehensive document that outlines the instructions for manufacturing a specific product batch. It. Master Batch Record In Pharmaceutical Industry.
From pharmablog.in
Reconciliation During Batch Manufacturing and Batch Packing SOP Master Batch Record In Pharmaceutical Industry Learn about the aims, objectives, key components,. The master batch record (mbr) is the foundation of all batch documentation. Master batch record (mbr) ensures consistency and accuracy in manufacturing processes. How does mbr help in pharma? Mbr is a comprehensive document that outlines the instructions for manufacturing a specific product batch. Learn how to prepare, review, approve, issue, maintain, and. Master Batch Record In Pharmaceutical Industry.
From www.deskera.com
What are Batch Manufacturing Records? Master Batch Record In Pharmaceutical Industry A master batch record (mbr), or master production record, is a comprehensive document that serves as the manufacturing. Learn about the aims, objectives, key components,. Learn how to prepare, review, approve, issue, maintain, and archive controlled master batch record (mbr) for pharmaceutical. Master batch record (mbr) ensures consistency and accuracy in manufacturing processes. It outlines every step of the manufacturing.. Master Batch Record In Pharmaceutical Industry.
From manoxblog.com
PHARMACEUTICAL BATCH MANUFACTURING RECORD Sample Download M A N O X Master Batch Record In Pharmaceutical Industry Learn about the aims, objectives, key components,. Mbr is a comprehensive document that outlines the instructions for manufacturing a specific product batch. The batch manufacturing record (bmr) is the compiled document created during the manufacturing process for each specific batch, based on the master batch record (mbr). Master batch record (mbr) ensures consistency and accuracy in manufacturing processes. A master. Master Batch Record In Pharmaceutical Industry.
From www.datacor.com
How to Prepare a Batch Manufacturing Record & Free Template (2022) Master Batch Record In Pharmaceutical Industry Mbr is a comprehensive document that outlines the instructions for manufacturing a specific product batch. A master batch record (mbr) contains the instructions, recipe or formula, and specific manufacturing process for a particular product. Learn how to prepare, review, approve, issue, maintain, and archive controlled master batch record (mbr) for pharmaceutical. Learn about the aims, objectives, key components,. A master. Master Batch Record In Pharmaceutical Industry.
From old.sermitsiaq.ag
Master Batch Record Template Master Batch Record In Pharmaceutical Industry Learn about the aims, objectives, key components,. The master batch record (mbr) is the foundation of all batch documentation. Learn how to prepare, review, approve, issue, maintain, and archive controlled master batch record (mbr) for pharmaceutical. How does mbr help in pharma? It ensures that each batch is produced in compliance Mbr is a comprehensive document that outlines the instructions. Master Batch Record In Pharmaceutical Industry.
From www.laafon.com
Importance of Batch Record Review in manufacturing of drugs Master Batch Record In Pharmaceutical Industry A master batch record (mbr) contains the instructions, recipe or formula, and specific manufacturing process for a particular product. The master batch record (mbr) is the foundation of all batch documentation. Master batch record (mbr) ensures consistency and accuracy in manufacturing processes. It ensures that each batch is produced in compliance It outlines every step of the manufacturing. The batch. Master Batch Record In Pharmaceutical Industry.
From www.pinterest.com
Formula & Batch Records Wholesale Supplies Plus Good manufacturing Master Batch Record In Pharmaceutical Industry Learn about the aims, objectives, key components,. Learn how to prepare, review, approve, issue, maintain, and archive controlled master batch record (mbr) for pharmaceutical. It outlines every step of the manufacturing. The master batch record (mbr) is the foundation of all batch documentation. Mbr is a comprehensive document that outlines the instructions for manufacturing a specific product batch. The batch. Master Batch Record In Pharmaceutical Industry.
From www.youtube.com
Batch Manufacturing Record (BMR) YouTube Master Batch Record In Pharmaceutical Industry The batch manufacturing record (bmr) is the compiled document created during the manufacturing process for each specific batch, based on the master batch record (mbr). The master batch record (mbr) is the foundation of all batch documentation. Learn how to prepare, review, approve, issue, maintain, and archive controlled master batch record (mbr) for pharmaceutical. It outlines every step of the. Master Batch Record In Pharmaceutical Industry.
From ciqa.net
What is a Master Batch Record (MBR) Versus a Batch Record (BR). Master Batch Record In Pharmaceutical Industry Learn how to prepare, review, approve, issue, maintain, and archive controlled master batch record (mbr) for pharmaceutical. A master batch record (mbr) contains the instructions, recipe or formula, and specific manufacturing process for a particular product. The batch manufacturing record (bmr) is the compiled document created during the manufacturing process for each specific batch, based on the master batch record. Master Batch Record In Pharmaceutical Industry.
From www.autoscribeinformatics.com
Pharmaceutical Manufacturing Flows Making the Complex Easy Master Batch Record In Pharmaceutical Industry It ensures that each batch is produced in compliance A master batch record (mbr), or master production record, is a comprehensive document that serves as the manufacturing. It outlines every step of the manufacturing. The batch manufacturing record (bmr) is the compiled document created during the manufacturing process for each specific batch, based on the master batch record (mbr). Mbr. Master Batch Record In Pharmaceutical Industry.
From manoxblog.com
Sample of Batch Manufacturing Record (BMR) Atorvastatin PDF Master Batch Record In Pharmaceutical Industry It ensures that each batch is produced in compliance How does mbr help in pharma? A master batch record (mbr), or master production record, is a comprehensive document that serves as the manufacturing. Learn about the aims, objectives, key components,. A master batch record (mbr) contains the instructions, recipe or formula, and specific manufacturing process for a particular product. It. Master Batch Record In Pharmaceutical Industry.
From www.mrpeasy.com
How to Keep Perfect Batch Records? MRPeasy Master Batch Record In Pharmaceutical Industry The batch manufacturing record (bmr) is the compiled document created during the manufacturing process for each specific batch, based on the master batch record (mbr). Learn how to prepare, review, approve, issue, maintain, and archive controlled master batch record (mbr) for pharmaceutical. How does mbr help in pharma? Master batch record (mbr) ensures consistency and accuracy in manufacturing processes. Learn. Master Batch Record In Pharmaceutical Industry.
From www.slideserve.com
PPT Batch Manufacturing Record and Master Formula Record PowerPoint Master Batch Record In Pharmaceutical Industry The batch manufacturing record (bmr) is the compiled document created during the manufacturing process for each specific batch, based on the master batch record (mbr). Master batch record (mbr) ensures consistency and accuracy in manufacturing processes. Learn about the aims, objectives, key components,. Mbr is a comprehensive document that outlines the instructions for manufacturing a specific product batch. A master. Master Batch Record In Pharmaceutical Industry.