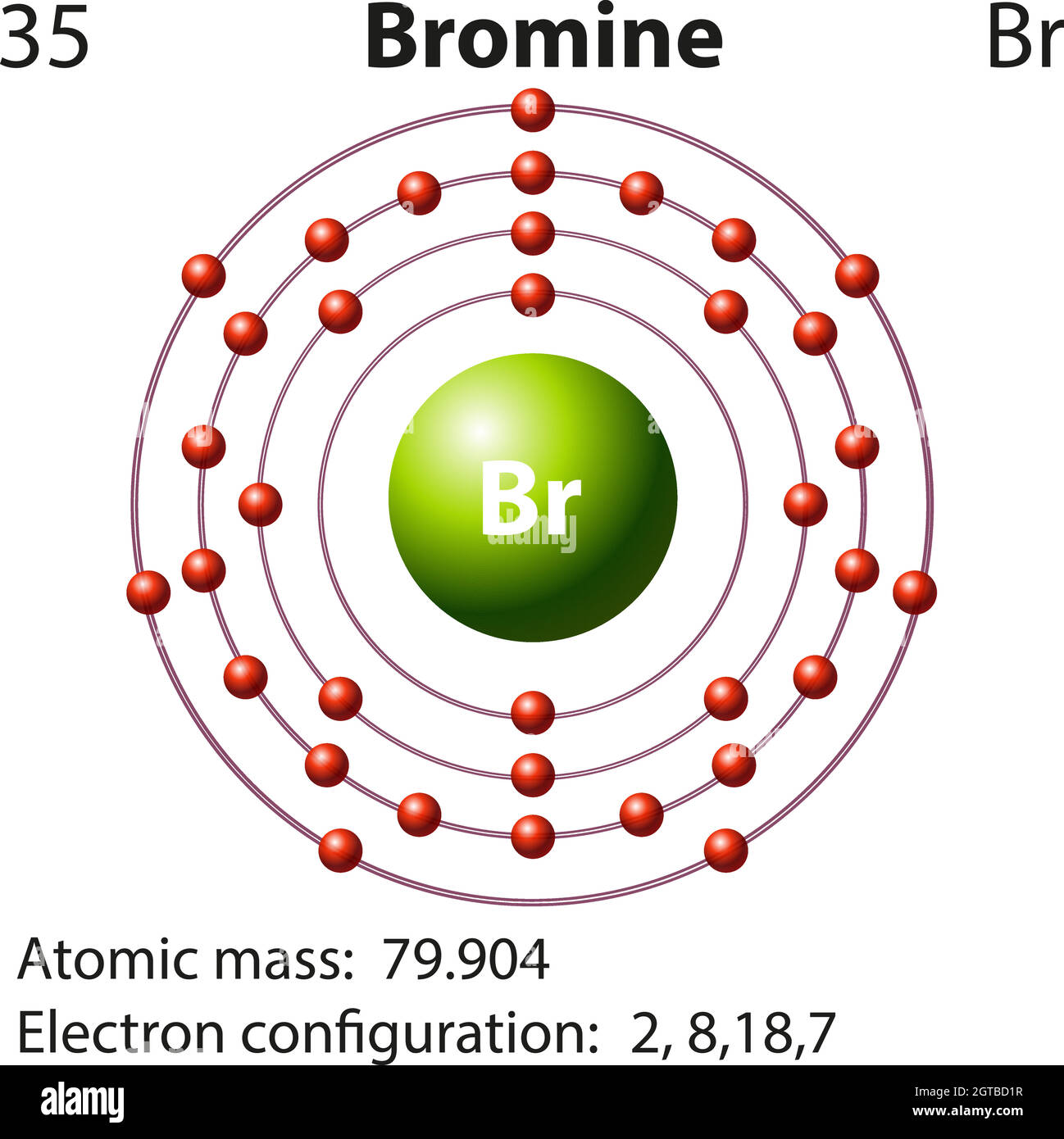
Bromine Has Blank Valence Electrons) . You can see that bromine has 7 valence electrons. The electrons which are distributed in the outermost shell of the. But for most of the transition. Valence electrons found in the s and p orbitals of the highest energy. Bromine has seven valence electrons. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. The valence electrons are be the 4s and 4p electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. How many valence electrons does bromine have? But how can you say that bromine has 7 valence electrons + how can you find these valence electrons?
from www.alamy.com
93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. The valence electrons are be the 4s and 4p electrons. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. But for most of the transition. How many valence electrons does bromine have? You can see that bromine has 7 valence electrons. But how can you say that bromine has 7 valence electrons + how can you find these valence electrons? Valence electrons found in the s and p orbitals of the highest energy. Bromine has seven valence electrons.
Symbol and electron diagram for Bromine Stock Vector Image & Art Alamy
Bromine Has Blank Valence Electrons) But how can you say that bromine has 7 valence electrons + how can you find these valence electrons? Bromine has seven valence electrons. You can see that bromine has 7 valence electrons. How many valence electrons does bromine have? Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. But for most of the transition. Valence electrons found in the s and p orbitals of the highest energy. But how can you say that bromine has 7 valence electrons + how can you find these valence electrons? The valence electrons are be the 4s and 4p electrons. The electrons which are distributed in the outermost shell of the. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that.
From hxethqwlt.blob.core.windows.net
How Many Valence Electrons Does Bromine Have at Karen Wallace blog Bromine Has Blank Valence Electrons) How many valence electrons does bromine have? You can see that bromine has 7 valence electrons. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. Bromine has seven valence electrons. The electrons which are distributed in the outermost shell of the. The total number of electrons present in. Bromine Has Blank Valence Electrons).
From valenceelectrons.com
How Many Valence Electrons Does SO2 (Sulfur Dioxide) Have? Bromine Has Blank Valence Electrons) The electrons which are distributed in the outermost shell of the. How many valence electrons does bromine have? 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Bromine has seven valence electrons. The electron configuration of an iron atom is 1s 2 2s 2 2p. Bromine Has Blank Valence Electrons).
From periodictable.me
Bromine Valence Electrons Bromine Valency (Br) Dot Diagram Bromine Has Blank Valence Electrons) Valence electrons found in the s and p orbitals of the highest energy. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. You can see that bromine has 7 valence electrons. The valence electrons are be the. Bromine Has Blank Valence Electrons).
From www.alamy.com
Chemist atom of Bromine diagram Stock Vector Image & Art Alamy Bromine Has Blank Valence Electrons) The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. Bromine has seven valence electrons. How many valence electrons does bromine have? But for most of the transition. The electron configuration of an iron atom is 1s 2. Bromine Has Blank Valence Electrons).
From proper-cooking.info
Bromine Valence Electrons Bromine Has Blank Valence Electrons) The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. Bromine has seven valence electrons. How many valence electrons does bromine have? The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in. Bromine Has Blank Valence Electrons).
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Has Blank Valence Electrons) The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. But how can you say. Bromine Has Blank Valence Electrons).
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine Has Blank Valence Electrons) How many valence electrons does bromine have? But for most of the transition. But how can you say that bromine has 7 valence electrons + how can you find these valence electrons? The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. Bromine has an electron configuration of 1s^2. Bromine Has Blank Valence Electrons).
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Has Blank Valence Electrons) But how can you say that bromine has 7 valence electrons + how can you find these valence electrons? But for most of the transition. Bromine has seven valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. How many valence electrons does bromine. Bromine Has Blank Valence Electrons).
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Has Blank Valence Electrons) Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. The electrons which are distributed in the outermost shell of the. But how can you say that bromine has 7 valence electrons + how can you find these valence electrons? The total number of electrons present in the valence shell of an atom are called valence electrons, and there are. Bromine Has Blank Valence Electrons).
From periodictable.me
2000pxElectron_configuration_bromine.svg Dynamic Periodic Table of Bromine Has Blank Valence Electrons) The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. You can see that bromine has 7. Bromine Has Blank Valence Electrons).
From material-properties.org
Bromine Periodic Table and Atomic Properties Bromine Has Blank Valence Electrons) The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. You can see that bromine has 7 valence electrons. Valence electrons found in the s and p orbitals of the highest energy. How many valence electrons does bromine have? 93 rows you may assume the valences of the chemical. Bromine Has Blank Valence Electrons).
From www.dreamstime.com
Bromine Chemical Element Symbol on Blue Bubble Background Stock Bromine Has Blank Valence Electrons) Valence electrons found in the s and p orbitals of the highest energy. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. But for most of the transition. How many valence electrons does bromine have? The valence. Bromine Has Blank Valence Electrons).
From valenceelectrons.com
How Many Valence Electrons Does Bromine (Br) Have? Bromine Has Blank Valence Electrons) Valence electrons found in the s and p orbitals of the highest energy. But for most of the transition. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. You can see that bromine has 7 valence electrons.. Bromine Has Blank Valence Electrons).
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Bromine Has Blank Valence Electrons) 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Valence electrons found in the s and p orbitals of the highest energy. You can see that bromine has 7 valence electrons. How many valence electrons does bromine have? Bromine has an electron configuration of 1s^2. Bromine Has Blank Valence Electrons).
From srkrkzbhxtdlh.blogspot.com
How Many Valence Electrons Does Bromine Have Bromine is a group viia Bromine Has Blank Valence Electrons) The electrons which are distributed in the outermost shell of the. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. The valence electrons are. Bromine Has Blank Valence Electrons).
From studyunlikeness.z21.web.core.windows.net
Which Has Seven Valence Electrons Bromine Has Blank Valence Electrons) How many valence electrons does bromine have? Bromine has seven valence electrons. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. Valence electrons found in the s and p orbitals of the highest energy. 93 rows you. Bromine Has Blank Valence Electrons).
From brainly.com
A calcium atom has {Fill in the blank} valence electrons. It needs to Bromine Has Blank Valence Electrons) The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. How many valence electrons does bromine have? The electrons which are distributed in the outermost shell of the. But how can you say that bromine has 7 valence electrons. Bromine Has Blank Valence Electrons).
From www.aiohotzgirl.com
Electron Configuration Bromine Chemical Element Electron Shell Bohr Bromine Has Blank Valence Electrons) The valence electrons are be the 4s and 4p electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. But for most of the transition. Valence electrons found in the s and p orbitals of the highest energy. You can see that bromine has 7. Bromine Has Blank Valence Electrons).
From www.youtube.com
How many valence electrons does bromine have? YouTube Bromine Has Blank Valence Electrons) Valence electrons found in the s and p orbitals of the highest energy. But for most of the transition. You can see that bromine has 7 valence electrons. How many valence electrons does bromine have? 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Bromine. Bromine Has Blank Valence Electrons).
From www.youtube.com
How many valence electrons does bromine have? YouTube Bromine Has Blank Valence Electrons) The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. But how can you say that bromine has 7 valence electrons + how can you find these valence electrons? Bromine has seven valence electrons. Valence electrons found in. Bromine Has Blank Valence Electrons).
From phootoscelebrities.com
How Many Valence Electrons Does Bromine Have? Bromine Has Blank Valence Electrons) 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. How many valence electrons. Bromine Has Blank Valence Electrons).
From www.youtube.com
Electron Configuration of Bromine, Br YouTube Bromine Has Blank Valence Electrons) The electrons which are distributed in the outermost shell of the. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. But for most of. Bromine Has Blank Valence Electrons).
From brainly.in
bromine valence electrons, Brainly.in Bromine Has Blank Valence Electrons) The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. The electrons which are distributed in the outermost shell of the. Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. How many valence electrons does bromine have? But for most of the transition. 93 rows you may assume. Bromine Has Blank Valence Electrons).
From www.numerade.com
SOLVED What is the valence electron configuration for the bromine atom? Bromine Has Blank Valence Electrons) How many valence electrons does bromine have? The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine.. Bromine Has Blank Valence Electrons).
From giohmdszv.blob.core.windows.net
Bromine Valence Electrons Number at Bruce Battin blog Bromine Has Blank Valence Electrons) The electrons which are distributed in the outermost shell of the. How many valence electrons does bromine have? The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. But for most of the transition. 93 rows you may assume the valences of the chemical elements—the number of electrons with. Bromine Has Blank Valence Electrons).
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine Has Blank Valence Electrons) You can see that bromine has 7 valence electrons. How many valence electrons does bromine have? But how can you say that bromine has 7 valence electrons + how can you find these valence electrons? Bromine has seven valence electrons. Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. 93 rows you may assume the valences of the chemical. Bromine Has Blank Valence Electrons).
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Has Blank Valence Electrons) The electrons which are distributed in the outermost shell of the. But how can you say that bromine has 7 valence electrons + how can you find these valence electrons? How many valence electrons does bromine have? Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. The valence electrons are be the 4s and 4p electrons. Bromine has seven. Bromine Has Blank Valence Electrons).
From www.alamy.com
Symbol and electron diagram for Bromine Stock Vector Image & Art Alamy Bromine Has Blank Valence Electrons) The valence electrons are be the 4s and 4p electrons. Valence electrons found in the s and p orbitals of the highest energy. But how can you say that bromine has 7 valence electrons + how can you find these valence electrons? How many valence electrons does bromine have? The electron configuration of an iron atom is 1s 2 2s. Bromine Has Blank Valence Electrons).
From www.chegg.com
Solved How many valence electrons are there in the bromine Bromine Has Blank Valence Electrons) The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. Valence electrons found in the s and p orbitals of the highest energy. The electrons which are distributed in the outermost shell of the. Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. You can see that bromine. Bromine Has Blank Valence Electrons).
From brainly.com
A calcium atom has {Fill in the blank} valence electrons. It needs to Bromine Has Blank Valence Electrons) But for most of the transition. How many valence electrons does bromine have? You can see that bromine has 7 valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. But how can you say that bromine has 7 valence electrons + how can. Bromine Has Blank Valence Electrons).
From fyohsioty.blob.core.windows.net
Neutral Bromine Atom Have Valence Electrons at Adriana Wix blog Bromine Has Blank Valence Electrons) 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The electrons which are distributed in the outermost shell of the. How many valence electrons does bromine have? Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. The electron configuration of an iron atom is. Bromine Has Blank Valence Electrons).
From socratic.org
Why does fluorine have a higher ionization energy than bromine? Socratic Bromine Has Blank Valence Electrons) But how can you say that bromine has 7 valence electrons + how can you find these valence electrons? Valence electrons found in the s and p orbitals of the highest energy. How many valence electrons does bromine have? You can see that bromine has 7 valence electrons. The valence electrons are be the 4s and 4p electrons. The total. Bromine Has Blank Valence Electrons).
From www.numerade.com
SOLVED 5. lodine is a oxidizing agent than bromine because the valence Bromine Has Blank Valence Electrons) Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. Bromine has seven valence electrons. The electrons which are distributed in the outermost shell of the. How many valence electrons does bromine have? You can see that bromine has 7 valence electrons. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6. Bromine Has Blank Valence Electrons).
From www.alamy.com
Bromine Chemical Element Graphic for Science Designs Stock Vector Image Bromine Has Blank Valence Electrons) The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. You can see that. Bromine Has Blank Valence Electrons).
From www.youtube.com
How to Find the Valence Electrons for Bromine (Br) YouTube Bromine Has Blank Valence Electrons) The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. Valence electrons found in the s and p orbitals of the highest energy. Bromine has seven valence electrons. How many valence electrons does bromine have? The electrons which are. Bromine Has Blank Valence Electrons).