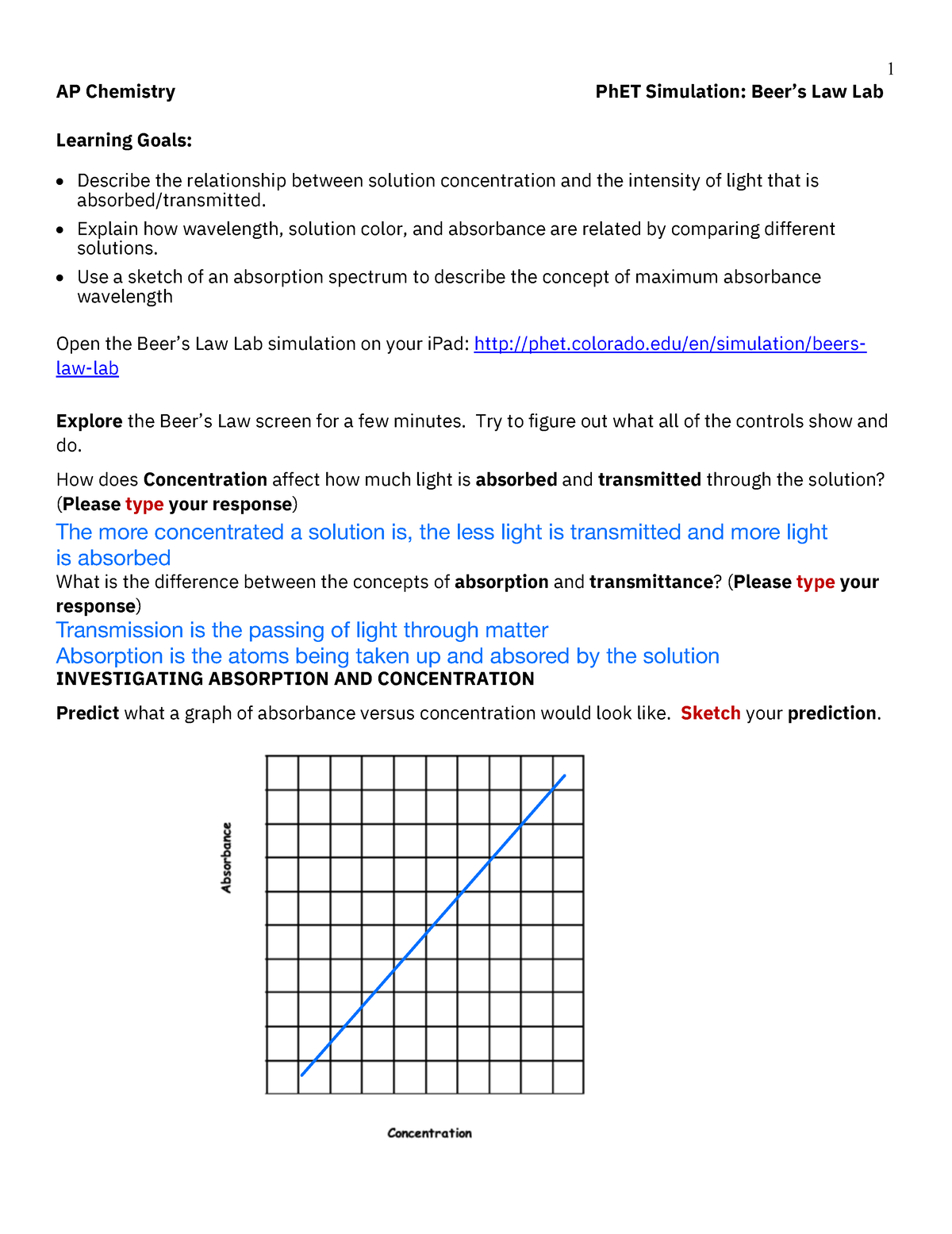
Beer's Law Hypothesis . the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The attenuation of light occurs as a result of distance through solution or increasing concentration. The premise is that a light beam becomes weaker as it passes through a chemical solution. a demonstration and simple questions that model the scientific approach are included to encourage students to think about. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,.
from www.studocu.com
The attenuation of light occurs as a result of distance through solution or increasing concentration. The premise is that a light beam becomes weaker as it passes through a chemical solution. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. a demonstration and simple questions that model the scientific approach are included to encourage students to think about. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,.
Beers Law Lab Ph ET Student Sheet AP Chemistry PhET Simulation Beer
Beer's Law Hypothesis the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. a demonstration and simple questions that model the scientific approach are included to encourage students to think about. The attenuation of light occurs as a result of distance through solution or increasing concentration. The premise is that a light beam becomes weaker as it passes through a chemical solution. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,.
From rrennenextanner.blogspot.com
Beer Lambert Law Ppt rrennenexTanner Beer's Law Hypothesis beer's law states that a chemical solution's concentration is directly proportional to its light absorption. a demonstration and simple questions that model the scientific approach are included to encourage students to think about. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. since the concentration, path length and molar. Beer's Law Hypothesis.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law Hypothesis The premise is that a light beam becomes weaker as it passes through a chemical solution. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. The attenuation of light occurs as a result of distance through solution or increasing concentration. beer’s law, in spectroscopy, a. Beer's Law Hypothesis.
From www.chegg.com
Solved Part 1 Beer's Law and Mass Absorption Coefficients Beer's Law Hypothesis beer's law states that a chemical solution's concentration is directly proportional to its light absorption. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. The attenuation of light occurs as a result of distance through solution or increasing concentration. the amount of light that a. Beer's Law Hypothesis.
From www.numerade.com
SOLVED Consider the Beer's Law equation A = εbc. Match the variable Beer's Law Hypothesis in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. The attenuation of light occurs as a result of distance through solution or. Beer's Law Hypothesis.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Beer's Law Hypothesis The premise is that a light beam becomes weaker as it passes through a chemical solution. The attenuation of light occurs as a result of distance through solution or increasing concentration. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. the amount of light that a species absorbs in a spectroscopic. Beer's Law Hypothesis.
From www.coursehero.com
[Solved] What factors are included in the Beer's law expression for Beer's Law Hypothesis in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy. Beer's Law Hypothesis.
From facts.net
13 Surprising Facts About BeerLambert Law Beer's Law Hypothesis beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. The premise is that a light beam becomes weaker as it passes through a chemical solution. The attenuation of light occurs as a result of distance through solution or increasing concentration. a demonstration and simple questions that model the scientific approach are. Beer's Law Hypothesis.
From plot.ly
Beer's Law Plot scatter chart made by Java789 plotly Beer's Law Hypothesis the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an. Beer's Law Hypothesis.
From www.chegg.com
Solved Describe what each term in the Beer's Law equation Beer's Law Hypothesis The premise is that a light beam becomes weaker as it passes through a chemical solution. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. The attenuation of light occurs as a result of distance through solution or increasing concentration. beer's law states that a. Beer's Law Hypothesis.
From goformative.com
Beer's Law Simulation for AP Chemistry Carson Dobrin Library Formative Beer's Law Hypothesis in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. The attenuation of light occurs as a result of distance through solution or increasing concentration. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. The premise is. Beer's Law Hypothesis.
From dxokacddk.blob.core.windows.net
Beer's Law Absorption at Ellen Day blog Beer's Law Hypothesis a demonstration and simple questions that model the scientific approach are included to encourage students to think about. The premise is that a light beam becomes weaker as it passes through a chemical solution. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. since the concentration, path length and molar absorptivity. Beer's Law Hypothesis.
From www.chegg.com
Solved Describe what each term in the Beer's Law equation Beer's Law Hypothesis since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. a demonstration and simple questions that model the scientific approach are included to encourage students to think about. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The premise is. Beer's Law Hypothesis.
From thechemistrynotes.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer's Law Hypothesis the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. since the concentration, path length and molar absorptivity are all directly. Beer's Law Hypothesis.
From www.studocu.com
Beer's law prelab Pre laboratory assignment for Beer's Law Beer's Law Hypothesis The premise is that a light beam becomes weaker as it passes through a chemical solution. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer's Law Hypothesis.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples Beer's Law Hypothesis The attenuation of light occurs as a result of distance through solution or increasing concentration. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number. Beer's Law Hypothesis.
From www.chegg.com
Solved Give the equation for Beer's Law and define the 4 Beer's Law Hypothesis in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to. Beer's Law Hypothesis.
From www.numerade.com
SOLVED The BeerLambert law relates the absorbance A of a solution to Beer's Law Hypothesis beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of. Beer's Law Hypothesis.
From dxowmggun.blob.core.windows.net
LambertBeer's Law Was Described To A General Equation at Thomas Partin Beer's Law Hypothesis a demonstration and simple questions that model the scientific approach are included to encourage students to think about. The attenuation of light occurs as a result of distance through solution or increasing concentration. The premise is that a light beam becomes weaker as it passes through a chemical solution. beer’s law, in spectroscopy, a relation concerning the absorption. Beer's Law Hypothesis.
From brainly.in
Beer lambert law formula Brainly.in Beer's Law Hypothesis The premise is that a light beam becomes weaker as it passes through a chemical solution. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. a. Beer's Law Hypothesis.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Hypothesis The premise is that a light beam becomes weaker as it passes through a chemical solution. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. a demonstration and simple questions that model the scientific approach are included to encourage students to think about.. Beer's Law Hypothesis.
From www.studocu.com
Beers Law Lab Ph ET Student Sheet AP Chemistry PhET Simulation Beer Beer's Law Hypothesis The attenuation of light occurs as a result of distance through solution or increasing concentration. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. The premise is that a light beam becomes weaker as it passes through a chemical solution. the amount of light that a. Beer's Law Hypothesis.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download Beer's Law Hypothesis beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The premise is that a light beam becomes weaker as it passes through a chemical solution. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. in spectroscopy, beer’s law. Beer's Law Hypothesis.
From goformative.com
Beer's Law Simulation for AP Chemistry Carson Dobrin Library Formative Beer's Law Hypothesis a demonstration and simple questions that model the scientific approach are included to encourage students to think about. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. . Beer's Law Hypothesis.
From ecency.com
Spectrophotometry Simplified The BeerLambert Law in Spectrophotom... Beer's Law Hypothesis beer's law states that a chemical solution's concentration is directly proportional to its light absorption. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. The attenuation of light occurs as a result of distance through solution or increasing concentration. since the concentration,. Beer's Law Hypothesis.
From calculatorghw.blogspot.com
Beer Lambert Law Calculator CALCULATOR GHW Beer's Law Hypothesis a demonstration and simple questions that model the scientific approach are included to encourage students to think about. The premise is that a light beam becomes weaker as it passes through a chemical solution. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. the amount. Beer's Law Hypothesis.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law Hypothesis a demonstration and simple questions that model the scientific approach are included to encourage students to think about. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The premise is that a light beam becomes weaker as it passes through a chemical solution. since the concentration, path length and molar absorptivity. Beer's Law Hypothesis.
From www.chegg.com
A Beer's Law experiment yielded the following data Beer's Law Hypothesis the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. a demonstration and simple questions that model the scientific approach are included to encourage students to think about. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. The. Beer's Law Hypothesis.
From www.youtube.com
Exp. 18 Spectrophotometric Analysis Concentration of a Solution Beer's Law Hypothesis beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. The attenuation of light occurs as a result of distance through solution or increasing concentration. The premise is that a light. Beer's Law Hypothesis.
From studybreathings.z21.web.core.windows.net
How To Calculate Absorbance Beer's Law Hypothesis the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. The premise is that a light beam becomes weaker as it passes through a chemical solution. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of. Beer's Law Hypothesis.
From studylib.net
A Beer's Law Experiment Introduction Beer's Law Hypothesis the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. The premise is that a light beam becomes weaker as it passes through a chemical solution. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of. Beer's Law Hypothesis.
From www.chegg.com
Solved BEERS LAWTransmi COLORIMETRYBEER'S LAW LAB REPORT Beer's Law Hypothesis the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer's Law Hypothesis.
From www.studocu.com
PreLab 3 Determining the concentration of a solution using beers law Beer's Law Hypothesis The premise is that a light beam becomes weaker as it passes through a chemical solution. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. The attenuation of. Beer's Law Hypothesis.
From www.researchgate.net
Validation of the LambertBeer law. Values of α associated with Beer's Law Hypothesis The premise is that a light beam becomes weaker as it passes through a chemical solution. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. a demonstration and simple questions. Beer's Law Hypothesis.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer's Law Hypothesis since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. the amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an. Beer's Law Hypothesis.
From studylib.net
LambertBeer Law Beer's Law Hypothesis in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. a demonstration and simple questions that model the scientific approach are included to encourage students to think about. The premise is that a light beam becomes weaker as it passes through a chemical solution.. Beer's Law Hypothesis.