What Is A Extracellular Buffer System . It consists of a solution of a weak acid and. buffer systems a buffer is a system that resists a change in ph. A buffer (or buffered) solution is one that resists a. This is responsible for about 80% of extracellular. the mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. definition of buffering. a variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the. intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. extracellular buffers include bicarbonate and ammonia, whereas proteins and phosphates act as intracellular buffers.
from www.youtube.com
extracellular buffers include bicarbonate and ammonia, whereas proteins and phosphates act as intracellular buffers. definition of buffering. It consists of a solution of a weak acid and. This is responsible for about 80% of extracellular. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. the mechanism involves a buffer, a solution that resists dramatic changes in ph. buffer systems a buffer is a system that resists a change in ph. a variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the. A buffer (or buffered) solution is one that resists a.
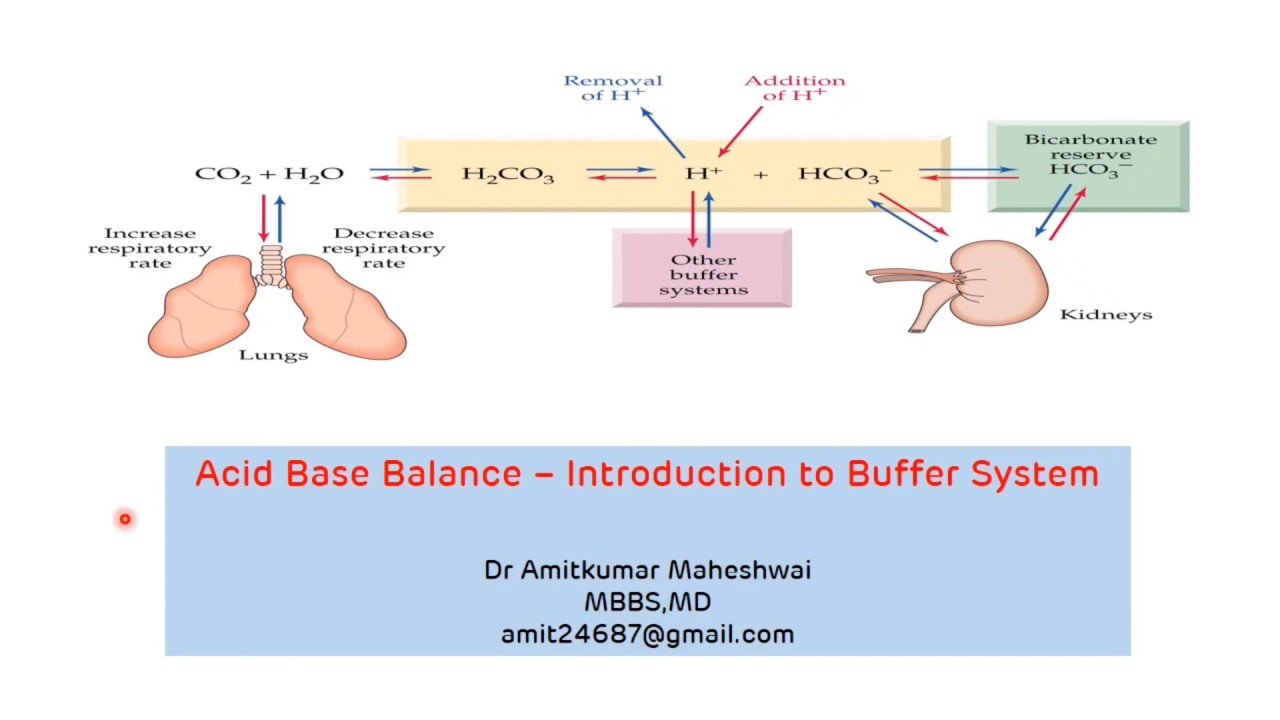
Introduction to Buffer System Regulation of pH Acid Base Balance
What Is A Extracellular Buffer System This is responsible for about 80% of extracellular. extracellular buffers include bicarbonate and ammonia, whereas proteins and phosphates act as intracellular buffers. buffer systems a buffer is a system that resists a change in ph. intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. A buffer (or buffered) solution is one that resists a. This is responsible for about 80% of extracellular. a variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the. It consists of a solution of a weak acid and. definition of buffering. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. the mechanism involves a buffer, a solution that resists dramatic changes in ph.
From www.slideserve.com
PPT Acid Base Balance PowerPoint Presentation, free download ID2413658 What Is A Extracellular Buffer System a variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the. extracellular buffers include bicarbonate and ammonia, whereas proteins and phosphates act as intracellular buffers. This is responsible for about 80% of extracellular. definition of buffering. intracellular and extracellular buffers are the most immediate mechanism of defense. What Is A Extracellular Buffer System.
From exojbdfvv.blob.core.windows.net
What Is Biological Buffer System at Maureen Masters blog What Is A Extracellular Buffer System This is responsible for about 80% of extracellular. a variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the. extracellular buffers include bicarbonate and ammonia, whereas proteins and phosphates act as intracellular buffers. A buffer is a solution that can resist ph change upon the addition of an acidic. What Is A Extracellular Buffer System.
From www.slideshare.net
Buffer system What Is A Extracellular Buffer System the mechanism involves a buffer, a solution that resists dramatic changes in ph. This is responsible for about 80% of extracellular. buffer systems a buffer is a system that resists a change in ph. A buffer (or buffered) solution is one that resists a. a variety of buffering systems permits blood and other bodily fluids to maintain. What Is A Extracellular Buffer System.
From www.slideserve.com
PPT Buffer Systems of the Body PowerPoint Presentation, free download What Is A Extracellular Buffer System the mechanism involves a buffer, a solution that resists dramatic changes in ph. a variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the. A buffer (or buffered) solution is one that resists a. extracellular buffers include bicarbonate and ammonia, whereas proteins and phosphates act as intracellular buffers.. What Is A Extracellular Buffer System.
From poorvi77.blogspot.com
Poorveez ParadyzMedimuseion major buffer systems in the body What Is A Extracellular Buffer System buffer systems a buffer is a system that resists a change in ph. the mechanism involves a buffer, a solution that resists dramatic changes in ph. a variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the. It consists of a solution of a weak acid and. A. What Is A Extracellular Buffer System.
From slidetodoc.com
Circulating portion of the extracellular fluid 25 of What Is A Extracellular Buffer System This is responsible for about 80% of extracellular. buffer systems a buffer is a system that resists a change in ph. A buffer (or buffered) solution is one that resists a. intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. A buffer is a solution that can resist ph change upon the. What Is A Extracellular Buffer System.
From exojbdfvv.blob.core.windows.net
What Is Biological Buffer System at Maureen Masters blog What Is A Extracellular Buffer System It consists of a solution of a weak acid and. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. This is responsible for about 80% of extracellular. buffer systems a buffer is a. What Is A Extracellular Buffer System.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance What Is A Extracellular Buffer System definition of buffering. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. extracellular buffers include bicarbonate and ammonia, whereas proteins and phosphates act as intracellular buffers. This is responsible for about 80% of extracellular. A buffer (or buffered) solution is one that resists a. the mechanism involves. What Is A Extracellular Buffer System.
From www.youtube.com
Chemical Buffers protein buffer, phosphate buffer system and What Is A Extracellular Buffer System extracellular buffers include bicarbonate and ammonia, whereas proteins and phosphates act as intracellular buffers. definition of buffering. A buffer (or buffered) solution is one that resists a. intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. buffer systems a buffer is a system that resists a change in ph. . What Is A Extracellular Buffer System.
From www.pinterest.ca
bicarbonate buffer system, example of multiple equilibria Teaching What Is A Extracellular Buffer System definition of buffering. a variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the. extracellular buffers include bicarbonate and ammonia, whereas proteins and phosphates act as intracellular buffers. It consists of a solution of a weak acid and. A buffer (or buffered) solution is one that resists a.. What Is A Extracellular Buffer System.
From www.slideserve.com
PPT Physiological system of blood. Functional importance of blood What Is A Extracellular Buffer System extracellular buffers include bicarbonate and ammonia, whereas proteins and phosphates act as intracellular buffers. A buffer (or buffered) solution is one that resists a. intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. This is responsible for about 80% of extracellular. a variety of buffering systems permits blood and other bodily. What Is A Extracellular Buffer System.
From www.slideserve.com
PPT Homeostasis PowerPoint Presentation, free download ID1138529 What Is A Extracellular Buffer System buffer systems a buffer is a system that resists a change in ph. definition of buffering. intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. A buffer (or buffered) solution is one that resists a. the mechanism involves a buffer, a solution that resists dramatic changes in ph. extracellular. What Is A Extracellular Buffer System.
From www.slideserve.com
PPT Integrative Physiology PowerPoint Presentation, free download What Is A Extracellular Buffer System This is responsible for about 80% of extracellular. buffer systems a buffer is a system that resists a change in ph. intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It consists of. What Is A Extracellular Buffer System.
From www.online-sciences.com
Body fluid exchange, The electrical characteristic of the cells What Is A Extracellular Buffer System A buffer (or buffered) solution is one that resists a. a variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the. This is responsible for about 80% of extracellular. definition of buffering. buffer systems a buffer is a system that resists a change in ph. the mechanism. What Is A Extracellular Buffer System.
From quizlet.com
buffering system Diagram Quizlet What Is A Extracellular Buffer System extracellular buffers include bicarbonate and ammonia, whereas proteins and phosphates act as intracellular buffers. intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. buffer systems a buffer is a system that resists a change in ph. definition of buffering. It consists of a solution of a weak acid and. A. What Is A Extracellular Buffer System.
From www.zuniv.net
New Human Physiology Ch 17 What Is A Extracellular Buffer System definition of buffering. extracellular buffers include bicarbonate and ammonia, whereas proteins and phosphates act as intracellular buffers. It consists of a solution of a weak acid and. This is responsible for about 80% of extracellular. the mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer (or buffered) solution is one that resists. What Is A Extracellular Buffer System.
From www.youtube.com
Buffer action in the blood YouTube What Is A Extracellular Buffer System the mechanism involves a buffer, a solution that resists dramatic changes in ph. This is responsible for about 80% of extracellular. definition of buffering. a variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the. extracellular buffers include bicarbonate and ammonia, whereas proteins and phosphates act as. What Is A Extracellular Buffer System.
From www.anaesthesiajournal.co.uk
Renal physiology acidbase balance Anaesthesia & Intensive Care Medicine What Is A Extracellular Buffer System extracellular buffers include bicarbonate and ammonia, whereas proteins and phosphates act as intracellular buffers. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. definition of buffering. the mechanism involves a buffer, a solution that resists dramatic changes in ph. a variety of buffering systems permits blood. What Is A Extracellular Buffer System.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID What Is A Extracellular Buffer System A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. buffer systems a buffer is a system that resists a change in ph. intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. the mechanism involves a buffer, a solution that resists dramatic. What Is A Extracellular Buffer System.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID What Is A Extracellular Buffer System It consists of a solution of a weak acid and. a variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the. the mechanism involves a buffer, a solution that resists dramatic changes in ph. extracellular buffers include bicarbonate and ammonia, whereas proteins and phosphates act as intracellular buffers.. What Is A Extracellular Buffer System.
From www.slideserve.com
PPT Buffer Systems of the Body PowerPoint Presentation, free download What Is A Extracellular Buffer System definition of buffering. This is responsible for about 80% of extracellular. buffer systems a buffer is a system that resists a change in ph. a variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the. the mechanism involves a buffer, a solution that resists dramatic changes in. What Is A Extracellular Buffer System.
From www.slideserve.com
PPT Fluid, Electrolyte, and AcidBase Balance PowerPoint Presentation What Is A Extracellular Buffer System the mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. buffer systems a buffer is a system that resists a change in ph. A buffer (or buffered) solution is one that resists a. a variety. What Is A Extracellular Buffer System.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent What Is A Extracellular Buffer System definition of buffering. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer (or buffered) solution is one that resists a. intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. This is responsible for about 80% of extracellular. a variety. What Is A Extracellular Buffer System.
From www.slideserve.com
PPT Fluid and Electrolytes Balance and Disturbance PowerPoint What Is A Extracellular Buffer System the mechanism involves a buffer, a solution that resists dramatic changes in ph. This is responsible for about 80% of extracellular. A buffer (or buffered) solution is one that resists a. definition of buffering. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. intracellular and extracellular buffers. What Is A Extracellular Buffer System.
From www.slideserve.com
PPT Fluids and Electrolytes PowerPoint Presentation, free download What Is A Extracellular Buffer System buffer systems a buffer is a system that resists a change in ph. intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. extracellular buffers include bicarbonate and ammonia, whereas proteins and phosphates act as intracellular buffers. the mechanism involves a buffer, a solution that resists dramatic changes in ph. This. What Is A Extracellular Buffer System.
From www.studocu.com
Biological Buffer Systems BIOLOGICAL BUFFER SYSTEMS Almost every What Is A Extracellular Buffer System It consists of a solution of a weak acid and. definition of buffering. a variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the. the mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer (or buffered) solution is one that resists a. A. What Is A Extracellular Buffer System.
From ar.inspiredpencil.com
Renal Buffer System What Is A Extracellular Buffer System a variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the. This is responsible for about 80% of extracellular. definition of buffering. buffer systems a buffer is a system that resists a change in ph. It consists of a solution of a weak acid and. extracellular buffers. What Is A Extracellular Buffer System.
From labpedia.net
Acidbase Balance Part 3 Respiratory Acidosis and Respiratory What Is A Extracellular Buffer System extracellular buffers include bicarbonate and ammonia, whereas proteins and phosphates act as intracellular buffers. a variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the. A buffer (or buffered) solution is one that resists a. intracellular and extracellular buffers are the most immediate mechanism of defense against changes. What Is A Extracellular Buffer System.
From www.wikidoc.org
Extracellular matrix wikidoc What Is A Extracellular Buffer System the mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer (or buffered) solution is one that resists a. It consists of a solution of a weak acid and. definition of buffering. extracellular buffers include bicarbonate and ammonia, whereas proteins and phosphates act as intracellular buffers. a variety of buffering systems permits. What Is A Extracellular Buffer System.
From www.slideserve.com
PPT Chapter 27 Fluid, Electrolyte and Acidbase Balance PowerPoint What Is A Extracellular Buffer System the mechanism involves a buffer, a solution that resists dramatic changes in ph. intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. buffer systems a buffer is a system that resists a change in ph. A buffer is a solution that can resist ph change upon the addition of an acidic. What Is A Extracellular Buffer System.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation What Is A Extracellular Buffer System a variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the. intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. the mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer (or buffered) solution is one that resists. What Is A Extracellular Buffer System.
From www.slideserve.com
PPT Fluid, Electrolyte, and AcidBase Balance PowerPoint Presentation What Is A Extracellular Buffer System a variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer (or buffered) solution is one that resists a. buffer systems a buffer is a system that resists. What Is A Extracellular Buffer System.
From www.slideserve.com
PPT AcidBase Balance PowerPoint Presentation, free download ID1994730 What Is A Extracellular Buffer System It consists of a solution of a weak acid and. A buffer (or buffered) solution is one that resists a. the mechanism involves a buffer, a solution that resists dramatic changes in ph. extracellular buffers include bicarbonate and ammonia, whereas proteins and phosphates act as intracellular buffers. intracellular and extracellular buffers are the most immediate mechanism of. What Is A Extracellular Buffer System.
From derangedphysiology.com
Buffering in acute respiratory acidbase disturbances Deranged Physiology What Is A Extracellular Buffer System A buffer (or buffered) solution is one that resists a. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. definition of buffering. This is responsible for about 80% of extracellular. intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. buffer systems. What Is A Extracellular Buffer System.
From abdominalkey.com
AcidBase Balance Roles of Chemical Buffers, Lungs, and Kidneys in What Is A Extracellular Buffer System extracellular buffers include bicarbonate and ammonia, whereas proteins and phosphates act as intracellular buffers. a variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the. definition of buffering. A buffer (or buffered) solution is one that resists a. This is responsible for about 80% of extracellular. A buffer. What Is A Extracellular Buffer System.