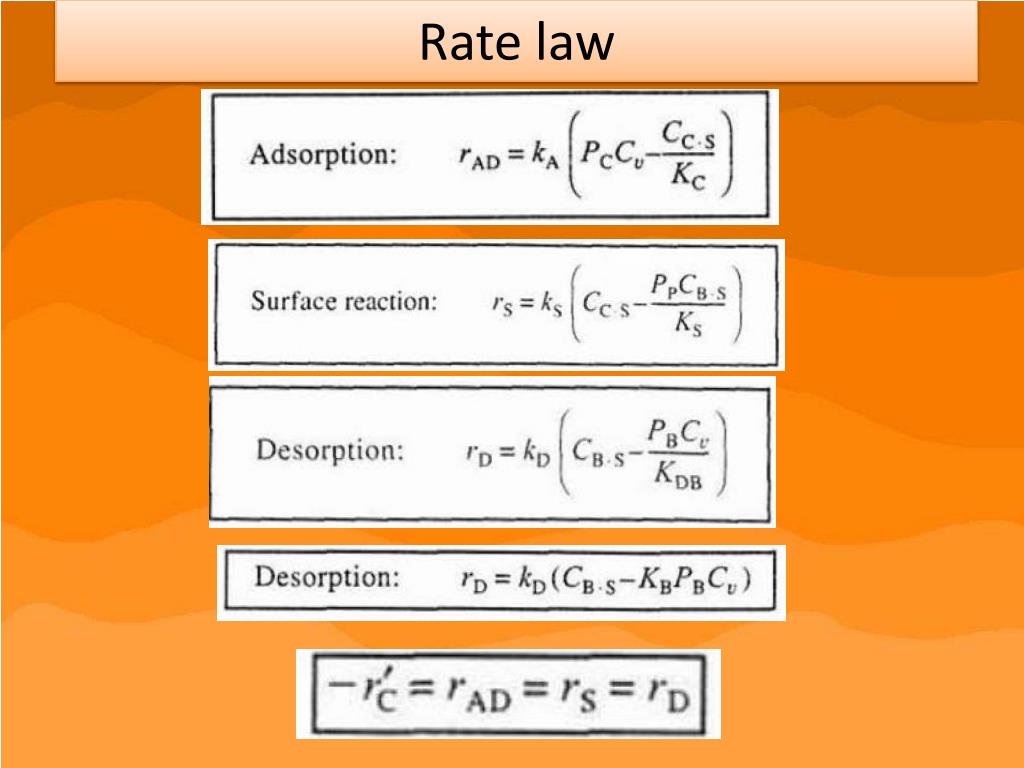
Catalysts Rate Equation . the results of such experiments are usually summarized in a rate equation or rate law for a given reaction. Investigate the rate of reaction by colour change. the rate equation shows the effect of changing the concentrations of the reactants on the rate of the reaction. A rate equation shows this effect mathematically. What about all the other things (like. revision notes on 5.2.1 rate equations for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. changing the concentration of substances taking part in a reaction usually changes the rate of the reaction. The rate of a chemical reaction can. Equations (1) and (3), for example,. the rate equation relates mathematically the rate of reaction to the concentration of the reactants. the rate of the reaction can then be calculated by determining the slope of a graph of concentration versus time. The greater the frequency of successful collisions between reactant.
from www.slideserve.com
The rate of a chemical reaction can. changing the concentration of substances taking part in a reaction usually changes the rate of the reaction. The greater the frequency of successful collisions between reactant. Equations (1) and (3), for example,. the results of such experiments are usually summarized in a rate equation or rate law for a given reaction. revision notes on 5.2.1 rate equations for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. A rate equation shows this effect mathematically. What about all the other things (like. the rate of the reaction can then be calculated by determining the slope of a graph of concentration versus time. Investigate the rate of reaction by colour change.
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint
Catalysts Rate Equation What about all the other things (like. the rate equation shows the effect of changing the concentrations of the reactants on the rate of the reaction. Investigate the rate of reaction by colour change. Equations (1) and (3), for example,. changing the concentration of substances taking part in a reaction usually changes the rate of the reaction. The greater the frequency of successful collisions between reactant. revision notes on 5.2.1 rate equations for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. the rate equation relates mathematically the rate of reaction to the concentration of the reactants. the results of such experiments are usually summarized in a rate equation or rate law for a given reaction. A rate equation shows this effect mathematically. What about all the other things (like. The rate of a chemical reaction can. the rate of the reaction can then be calculated by determining the slope of a graph of concentration versus time.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalysts Rate Equation The rate of a chemical reaction can. Investigate the rate of reaction by colour change. changing the concentration of substances taking part in a reaction usually changes the rate of the reaction. the rate of the reaction can then be calculated by determining the slope of a graph of concentration versus time. the rate equation shows the. Catalysts Rate Equation.
From www.youtube.com
R2.2.6 Intermediates and catalysts (HL) YouTube Catalysts Rate Equation Equations (1) and (3), for example,. Investigate the rate of reaction by colour change. revision notes on 5.2.1 rate equations for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. the rate equation shows the effect of changing the concentrations of the reactants on the rate of the reaction. the rate. Catalysts Rate Equation.
From www.youtube.com
Factors Affecting the Rate of Reaction Catalyst Rate of Reaction Catalysts Rate Equation Investigate the rate of reaction by colour change. What about all the other things (like. The rate of a chemical reaction can. The greater the frequency of successful collisions between reactant. the results of such experiments are usually summarized in a rate equation or rate law for a given reaction. changing the concentration of substances taking part in. Catalysts Rate Equation.
From exobghamq.blob.core.windows.net
Catalysts Chemical Equation at Joy Perry blog Catalysts Rate Equation revision notes on 5.2.1 rate equations for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. The rate of a chemical reaction can. A rate equation shows this effect mathematically. the results of such experiments are usually summarized in a rate equation or rate law for a given reaction. the rate. Catalysts Rate Equation.
From www.sasadoctor.com
Rate of reaction chemistry igcse Catalysts Rate Equation changing the concentration of substances taking part in a reaction usually changes the rate of the reaction. The rate of a chemical reaction can. Equations (1) and (3), for example,. The greater the frequency of successful collisions between reactant. the results of such experiments are usually summarized in a rate equation or rate law for a given reaction.. Catalysts Rate Equation.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub Catalysts Rate Equation Equations (1) and (3), for example,. changing the concentration of substances taking part in a reaction usually changes the rate of the reaction. the rate equation shows the effect of changing the concentrations of the reactants on the rate of the reaction. The greater the frequency of successful collisions between reactant. A rate equation shows this effect mathematically.. Catalysts Rate Equation.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalysts Rate Equation Equations (1) and (3), for example,. the results of such experiments are usually summarized in a rate equation or rate law for a given reaction. A rate equation shows this effect mathematically. The greater the frequency of successful collisions between reactant. What about all the other things (like. the rate equation shows the effect of changing the concentrations. Catalysts Rate Equation.
From www.youtube.com
Catalysts AP Chemistry Khan Academy YouTube Catalysts Rate Equation changing the concentration of substances taking part in a reaction usually changes the rate of the reaction. the rate equation shows the effect of changing the concentrations of the reactants on the rate of the reaction. the results of such experiments are usually summarized in a rate equation or rate law for a given reaction. revision. Catalysts Rate Equation.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalysts Rate Equation The rate of a chemical reaction can. Equations (1) and (3), for example,. the rate of the reaction can then be calculated by determining the slope of a graph of concentration versus time. The greater the frequency of successful collisions between reactant. the results of such experiments are usually summarized in a rate equation or rate law for. Catalysts Rate Equation.
From courses.lumenlearning.com
Catalysis Chemistry Catalysts Rate Equation the rate of the reaction can then be calculated by determining the slope of a graph of concentration versus time. revision notes on 5.2.1 rate equations for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. What about all the other things (like. the rate equation relates mathematically the rate of. Catalysts Rate Equation.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalysts Rate Equation Equations (1) and (3), for example,. the rate of the reaction can then be calculated by determining the slope of a graph of concentration versus time. the rate equation shows the effect of changing the concentrations of the reactants on the rate of the reaction. The greater the frequency of successful collisions between reactant. A rate equation shows. Catalysts Rate Equation.
From circuitdbplastered.z13.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalysts Rate Equation the rate equation shows the effect of changing the concentrations of the reactants on the rate of the reaction. revision notes on 5.2.1 rate equations for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. the rate equation relates mathematically the rate of reaction to the concentration of the reactants. A. Catalysts Rate Equation.
From www.reddit.com
Finding the rate law from a ratedetermining step that contains species Catalysts Rate Equation A rate equation shows this effect mathematically. revision notes on 5.2.1 rate equations for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. Equations (1) and (3), for example,. The greater the frequency of successful collisions between reactant. What about all the other things (like. the rate equation shows the effect of. Catalysts Rate Equation.
From www.slideserve.com
PPT Rates of Reactions PowerPoint Presentation, free download ID Catalysts Rate Equation The greater the frequency of successful collisions between reactant. changing the concentration of substances taking part in a reaction usually changes the rate of the reaction. revision notes on 5.2.1 rate equations for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. The rate of a chemical reaction can. the rate. Catalysts Rate Equation.
From jackwestin.com
Rate Processes Catalysts Rate Processes In Chemical Reactions Catalysts Rate Equation A rate equation shows this effect mathematically. the rate of the reaction can then be calculated by determining the slope of a graph of concentration versus time. The rate of a chemical reaction can. changing the concentration of substances taking part in a reaction usually changes the rate of the reaction. The greater the frequency of successful collisions. Catalysts Rate Equation.
From slideplayer.com
Controlling Reactions ppt download Catalysts Rate Equation The rate of a chemical reaction can. the rate of the reaction can then be calculated by determining the slope of a graph of concentration versus time. the results of such experiments are usually summarized in a rate equation or rate law for a given reaction. Equations (1) and (3), for example,. the rate equation relates mathematically. Catalysts Rate Equation.
From millerdidettioners.blogspot.com
How Does Particle Size Affect Reaction Rate Miller Didettioners Catalysts Rate Equation Investigate the rate of reaction by colour change. the results of such experiments are usually summarized in a rate equation or rate law for a given reaction. A rate equation shows this effect mathematically. The rate of a chemical reaction can. the rate equation relates mathematically the rate of reaction to the concentration of the reactants. revision. Catalysts Rate Equation.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalysts Rate Equation A rate equation shows this effect mathematically. The rate of a chemical reaction can. The greater the frequency of successful collisions between reactant. revision notes on 5.2.1 rate equations for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. the rate of the reaction can then be calculated by determining the slope. Catalysts Rate Equation.
From ar.inspiredpencil.com
Catalyst Equation Catalysts Rate Equation Equations (1) and (3), for example,. Investigate the rate of reaction by colour change. A rate equation shows this effect mathematically. What about all the other things (like. revision notes on 5.2.1 rate equations for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. The rate of a chemical reaction can. The greater. Catalysts Rate Equation.
From www.slideserve.com
PPT Reaction mechanisms and catalysts PowerPoint Presentation, free Catalysts Rate Equation the rate equation shows the effect of changing the concentrations of the reactants on the rate of the reaction. The greater the frequency of successful collisions between reactant. the rate of the reaction can then be calculated by determining the slope of a graph of concentration versus time. changing the concentration of substances taking part in a. Catalysts Rate Equation.
From www.slideserve.com
PPT Reaction Mechanisms PowerPoint Presentation, free download ID Catalysts Rate Equation the results of such experiments are usually summarized in a rate equation or rate law for a given reaction. Investigate the rate of reaction by colour change. the rate equation shows the effect of changing the concentrations of the reactants on the rate of the reaction. A rate equation shows this effect mathematically. the rate equation relates. Catalysts Rate Equation.
From byjus.com
How does a catalyst increase the rate of a reaction? Catalysts Rate Equation the rate equation shows the effect of changing the concentrations of the reactants on the rate of the reaction. The greater the frequency of successful collisions between reactant. The rate of a chemical reaction can. the rate of the reaction can then be calculated by determining the slope of a graph of concentration versus time. Investigate the rate. Catalysts Rate Equation.
From exobghamq.blob.core.windows.net
Catalysts Chemical Equation at Joy Perry blog Catalysts Rate Equation A rate equation shows this effect mathematically. Equations (1) and (3), for example,. Investigate the rate of reaction by colour change. revision notes on 5.2.1 rate equations for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. the rate equation shows the effect of changing the concentrations of the reactants on the. Catalysts Rate Equation.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalysts Rate Equation the rate equation relates mathematically the rate of reaction to the concentration of the reactants. What about all the other things (like. A rate equation shows this effect mathematically. revision notes on 5.2.1 rate equations for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. The rate of a chemical reaction can.. Catalysts Rate Equation.
From www.nagwa.com
Question Video Describing the Effect of Catalysts on Reaction Rates Catalysts Rate Equation the rate equation relates mathematically the rate of reaction to the concentration of the reactants. changing the concentration of substances taking part in a reaction usually changes the rate of the reaction. Investigate the rate of reaction by colour change. the rate of the reaction can then be calculated by determining the slope of a graph of. Catalysts Rate Equation.
From chemistryguru.com.sg
Rate Equation and Order of Reaction Catalysts Rate Equation What about all the other things (like. A rate equation shows this effect mathematically. The rate of a chemical reaction can. Investigate the rate of reaction by colour change. the results of such experiments are usually summarized in a rate equation or rate law for a given reaction. Equations (1) and (3), for example,. The greater the frequency of. Catalysts Rate Equation.
From exotukoyg.blob.core.windows.net
Catalyst Reaction Chemical Equation at Glenn Leopard blog Catalysts Rate Equation The greater the frequency of successful collisions between reactant. the results of such experiments are usually summarized in a rate equation or rate law for a given reaction. the rate equation shows the effect of changing the concentrations of the reactants on the rate of the reaction. changing the concentration of substances taking part in a reaction. Catalysts Rate Equation.
From www.slideserve.com
PPT Catalysis & Catalysts PowerPoint Presentation, free download ID Catalysts Rate Equation revision notes on 5.2.1 rate equations for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. The rate of a chemical reaction can. the rate of the reaction can then be calculated by determining the slope of a graph of concentration versus time. the rate equation shows the effect of changing. Catalysts Rate Equation.
From www.slideshare.net
8.1 rate law Catalysts Rate Equation the rate of the reaction can then be calculated by determining the slope of a graph of concentration versus time. the results of such experiments are usually summarized in a rate equation or rate law for a given reaction. Equations (1) and (3), for example,. A rate equation shows this effect mathematically. the rate equation shows the. Catalysts Rate Equation.
From www.slideserve.com
PPT Rate laws (2) PowerPoint Presentation, free download ID2351800 Catalysts Rate Equation Equations (1) and (3), for example,. The greater the frequency of successful collisions between reactant. The rate of a chemical reaction can. A rate equation shows this effect mathematically. the results of such experiments are usually summarized in a rate equation or rate law for a given reaction. changing the concentration of substances taking part in a reaction. Catalysts Rate Equation.
From slideplayer.com
Catalysts Rates of Reactions. ppt download Catalysts Rate Equation A rate equation shows this effect mathematically. The rate of a chemical reaction can. What about all the other things (like. revision notes on 5.2.1 rate equations for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. Investigate the rate of reaction by colour change. changing the concentration of substances taking part. Catalysts Rate Equation.
From chemistryguru.com.sg
Rate Concentration Graph for Enzyme Catalysed Reaction Catalysts Rate Equation A rate equation shows this effect mathematically. the rate of the reaction can then be calculated by determining the slope of a graph of concentration versus time. The greater the frequency of successful collisions between reactant. changing the concentration of substances taking part in a reaction usually changes the rate of the reaction. Investigate the rate of reaction. Catalysts Rate Equation.
From www.studocu.com
3.7 Reaction Rates Catalysts & Equilibrium Lecture 28 catalysts Catalysts Rate Equation The rate of a chemical reaction can. changing the concentration of substances taking part in a reaction usually changes the rate of the reaction. What about all the other things (like. the rate equation relates mathematically the rate of reaction to the concentration of the reactants. revision notes on 5.2.1 rate equations for the aqa a level. Catalysts Rate Equation.
From www.slideserve.com
PPT Break PowerPoint Presentation, free download ID2714864 Catalysts Rate Equation the rate equation shows the effect of changing the concentrations of the reactants on the rate of the reaction. the rate equation relates mathematically the rate of reaction to the concentration of the reactants. The greater the frequency of successful collisions between reactant. the results of such experiments are usually summarized in a rate equation or rate. Catalysts Rate Equation.
From exobghamq.blob.core.windows.net
Catalysts Chemical Equation at Joy Perry blog Catalysts Rate Equation the rate equation shows the effect of changing the concentrations of the reactants on the rate of the reaction. A rate equation shows this effect mathematically. revision notes on 5.2.1 rate equations for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. The greater the frequency of successful collisions between reactant. Equations. Catalysts Rate Equation.