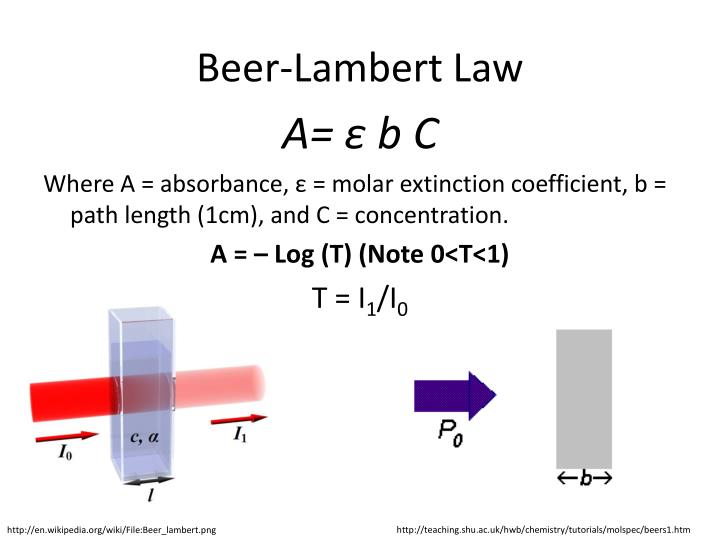
Beer's Law Home Experiment . “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called the. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. A = abc, where a is the absorbance of the solution, a is the molar. Explore how concentration and thickness affect light absorption and transmission in solutions using a virtual spectrophotometer. This relationship is known as beer’s law and is given by the equation: In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as.
from fyooddpjn.blob.core.windows.net
A = abc, where a is the absorbance of the solution, a is the molar. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. This relationship is called the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a. Explore how concentration and thickness affect light absorption and transmission in solutions using a virtual spectrophotometer. This relationship is known as beer’s law and is given by the equation: The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species.
Beer Lambert Law Enzyme Activity at Russell Bingaman blog
Beer's Law Home Experiment A = abc, where a is the absorbance of the solution, a is the molar. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. A = abc, where a is the absorbance of the solution, a is the molar. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. This relationship is known as beer’s law and is given by the equation: “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a. This relationship is called the. Explore how concentration and thickness affect light absorption and transmission in solutions using a virtual spectrophotometer.
From www.slideserve.com
PPT beers law lab experiment PowerPoint Presentation, free download Beer's Law Home Experiment Explore how concentration and thickness affect light absorption and transmission in solutions using a virtual spectrophotometer. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a. This relationship is called the. In most experiments, molar absorptivity (ε) and. Beer's Law Home Experiment.
From www.chegg.com
Solved PreLab Assignment Beer's Law This experiment covers Beer's Law Home Experiment Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can. Beer's Law Home Experiment.
From www.studocu.com
Beer's Law postlab Report Sheet of experiment about Beer's Law Beer's Law Home Experiment The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. “the thicker the glass, the darker the brew, the less the light that. Beer's Law Home Experiment.
From www.youtube.com
BeerLambert law in easy way YouTube Beer's Law Home Experiment Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. This relationship is called the. Explore how concentration and thickness affect light absorption and transmission in solutions using a virtual spectrophotometer. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which. Beer's Law Home Experiment.
From studylib.net
Lab 7 Beer's Law Beer's Law Home Experiment This relationship is known as beer’s law and is given by the equation: Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light they absorb and transmit. Beer's Law Home Experiment.
From www.youtube.com
Chemistry Laboratory Verification of BeerLambert Law and determine Beer's Law Home Experiment The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. This relationship is known as beer’s law and is given by the equation: Explore how concentration and thickness affect light absorption. Beer's Law Home Experiment.
From openlab.citytech.cuny.edu
Beer’s Law Biology 1101 Course Hub Beer's Law Home Experiment “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a. This relationship is called the. Explore how concentration and thickness affect light absorption and transmission in solutions using a virtual spectrophotometer. This relationship is known as beer’s law. Beer's Law Home Experiment.
From www.vernier.com
Beer's Law Investigations > Experiment 11 from Investigating Chemistry Beer's Law Home Experiment Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Explore how concentration and thickness affect light absorption and transmission in solutions using a virtual spectrophotometer. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. This relationship. Beer's Law Home Experiment.
From chemistrytalk.org
BeerLambert Law ChemTalk Beer's Law Home Experiment Explore how concentration and thickness affect light absorption and transmission in solutions using a virtual spectrophotometer. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a. Explore beer's law by creating colorful solutions and measuring light absorption and. Beer's Law Home Experiment.
From laboratorysciences.blogspot.com
Beer's Law The Laboratory Beer's Law Home Experiment The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. A = abc, where a is the absorbance of the solution, a is. Beer's Law Home Experiment.
From fyooddpjn.blob.core.windows.net
Beer Lambert Law Enzyme Activity at Russell Bingaman blog Beer's Law Home Experiment This relationship is called the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with. Beer's Law Home Experiment.
From www.youtube.com
Spectrophotometry and Beer's Law YouTube Beer's Law Home Experiment This relationship is called the. A = abc, where a is the absorbance of the solution, a is the molar. Explore how concentration and thickness affect light absorption and transmission in solutions using a virtual spectrophotometer. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known. Beer's Law Home Experiment.
From mckennaroscervantes.blogspot.com
Beer's Lambert Law Equation MckennarosCervantes Beer's Law Home Experiment Explore how concentration and thickness affect light absorption and transmission in solutions using a virtual spectrophotometer. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and. Beer's Law Home Experiment.
From www.youtube.com
Experiment 1 Verify LambertBeer's Law. YouTube Beer's Law Home Experiment A = abc, where a is the absorbance of the solution, a is the molar. This relationship is known as beer’s law and is given by the equation: Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Explore how concentration and thickness affect light. Beer's Law Home Experiment.
From www.youtube.com
Spectrophotometry BeerLambert Law. YouTube Beer's Law Home Experiment In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. This relationship is called the. This relationship is known as beer’s law and is given by the equation: Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. The amount of light that a species absorbs in. Beer's Law Home Experiment.
From www.chegg.com
A Beer's Law experiment yielded the following data Beer's Law Home Experiment Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Explore how concentration and thickness affect light absorption and transmission in solutions using a virtual spectrophotometer. This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can be. Beer's Law Home Experiment.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Home Experiment “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional. Beer's Law Home Experiment.
From www.softpedia.com
Download Beer's Law Lab 1.02 Beer's Law Home Experiment Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a. This relationship is. Beer's Law Home Experiment.
From www.youtube.com
Beer's Law YouTube Beer's Law Home Experiment A = abc, where a is the absorbance of the solution, a is the molar. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a. This relationship is called the. In most experiments, molar absorptivity (ε) and the. Beer's Law Home Experiment.
From www.youtube.com
Beer's Law at Home YouTube Beer's Law Home Experiment Explore how concentration and thickness affect light absorption and transmission in solutions using a virtual spectrophotometer. This relationship is called the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. A = abc, where a is the absorbance of the solution, a is the molar. “the thicker the glass, the darker the brew,. Beer's Law Home Experiment.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law Home Experiment A = abc, where a is the absorbance of the solution, a is the molar. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. Beer's Law Home Experiment.
From studylib.net
A Beer's Law Experiment Introduction Beer's Law Home Experiment Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called the. Explore how concentration and thickness affect light absorption and transmission in solutions using a virtual spectrophotometer.. Beer's Law Home Experiment.
From calculatorghw.blogspot.com
Beer Lambert Law Calculator CALCULATOR GHW Beer's Law Home Experiment This relationship is called the. Explore how concentration and thickness affect light absorption and transmission in solutions using a virtual spectrophotometer. This relationship is known as beer’s law and is given by the equation: Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. Since the concentration, path length and molar absorptivity are. Beer's Law Home Experiment.
From www.youtube.com
Experiment 13 Beer's Law YouTube Beer's Law Home Experiment Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Explore how concentration and thickness affect light absorption and transmission in solutions using a virtual spectrophotometer. This relationship is known as beer’s law and is given by the equation: “the thicker the glass, the darker. Beer's Law Home Experiment.
From www.studocu.com
Beer's Law Experiment Experiment 4 Beer’s Law. Purpose The purpose Beer's Law Home Experiment A = abc, where a is the absorbance of the solution, a is the molar. Explore how concentration and thickness affect light absorption and transmission in solutions using a virtual spectrophotometer. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. This relationship is called the. Since the concentration, path length and molar absorptivity. Beer's Law Home Experiment.
From www.youtube.com
Beer’s Law Experiment with Nickel(II) Sulfate YouTube Beer's Law Home Experiment Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In most experiments, molar absorptivity (ε) and the. Beer's Law Home Experiment.
From www.studocu.com
Beer's Law Lab Beer's Law, 20192020 academic year Experiment 4 Beer's Law Home Experiment A = abc, where a is the absorbance of the solution, a is the molar. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. This relationship is called the. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore. Beer's Law Home Experiment.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer's Law Home Experiment “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a. This relationship is known as beer’s law and is given by the equation: Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a. Beer's Law Home Experiment.
From www.youtube.com
Beer Lambert law derivation and usage YouTube Beer's Law Home Experiment This relationship is known as beer’s law and is given by the equation: Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. A = abc, where a is the absorbance of the solution, a is the molar. The amount of light that a species absorbs in a spectroscopic transition can be related. Beer's Law Home Experiment.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Beer's Law Home Experiment In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Explore how concentration and thickness affect light absorption and transmission in solutions using a virtual spectrophotometer. This relationship is known as beer’s law and is given by the equation: This relationship is called the. A = abc, where a is the absorbance of the. Beer's Law Home Experiment.
From studylib.net
Beer`s Law Lab Beer's Law Home Experiment The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Explore how concentration and thickness affect light absorption and transmission in solutions using a virtual spectrophotometer. This relationship is known as beer’s law and is given by the equation: A = abc, where a is the absorbance of. Beer's Law Home Experiment.
From study.com
BeerLambert Law Equation, Units & Examples Lesson Beer's Law Home Experiment The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. A = abc, where a is the absorbance of the solution, a is the molar. Explore how concentration and thickness affect. Beer's Law Home Experiment.
From www.youtube.com
Beer's Law Lab Procedure YouTube Beer's Law Home Experiment This relationship is called the. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The amount of light that a species. Beer's Law Home Experiment.
From studylib.net
Beer's Law Lab Beer's Law Home Experiment This relationship is known as beer’s law and is given by the equation: Explore beer's law by creating colorful solutions and measuring light absorption and transmission with a virtual spectrophotometer. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. A = abc, where a. Beer's Law Home Experiment.
From www.studocu.com
Beers law experiment 3 3 EXPERIMENT 3 BEER’S LAW OBJECTIVES • To Beer's Law Home Experiment Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. This relationship is known as beer’s law and is given by the equation: A = abc, where a is the absorbance of the solution, a is the molar. Explore beer's law by creating colorful solutions. Beer's Law Home Experiment.