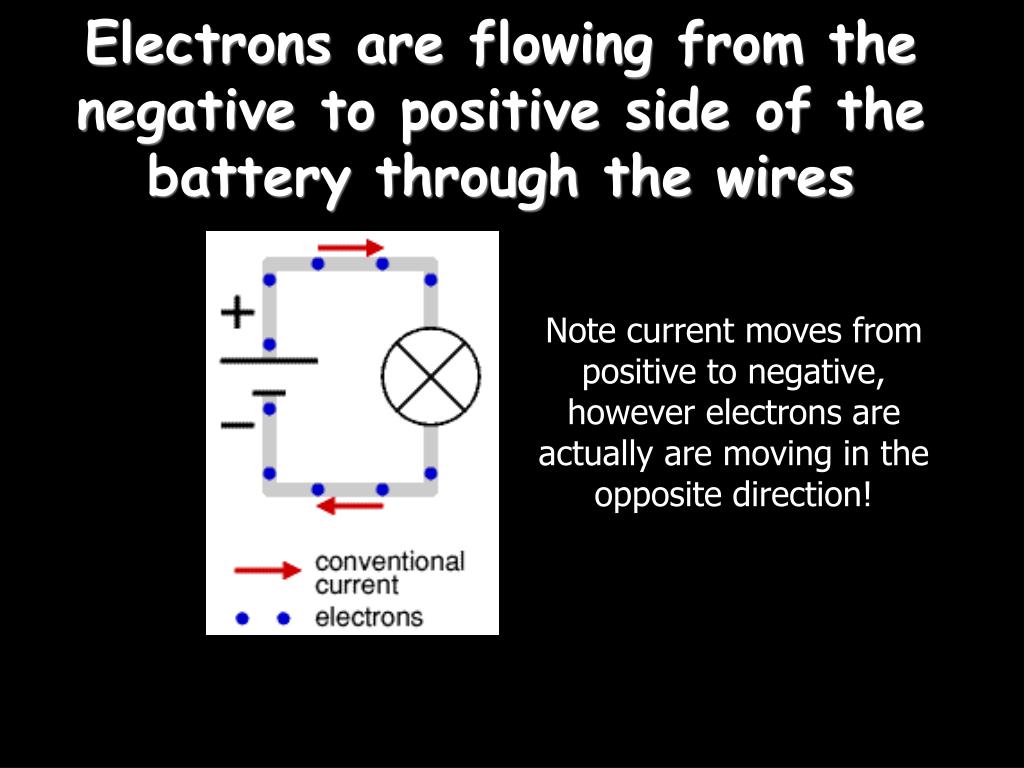
Electrons In A Battery Flow From . One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. It describes the method by which batteries generate electron flow as follows: When a device is connected to a battery — a light bulb or an electric circuit — chemical reactions occur on the electrodes that. There's essentially no flow of individual free electrons inside the battery. The electrons and ions flow because of the chemical reactions happening inside the battery—usually two of them going. The battery releases a few electrons via a chemical. However, there is a net flow of electrons since the ions include. The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. The flow of electrons provides an.
from www.slideserve.com
The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. The battery releases a few electrons via a chemical. One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. The electrons and ions flow because of the chemical reactions happening inside the battery—usually two of them going. It describes the method by which batteries generate electron flow as follows: When a device is connected to a battery — a light bulb or an electric circuit — chemical reactions occur on the electrodes that. The flow of electrons provides an. There's essentially no flow of individual free electrons inside the battery. However, there is a net flow of electrons since the ions include.
PPT Electricity PowerPoint Presentation, free download ID7008919
Electrons In A Battery Flow From The electrons and ions flow because of the chemical reactions happening inside the battery—usually two of them going. One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. There's essentially no flow of individual free electrons inside the battery. When a device is connected to a battery — a light bulb or an electric circuit — chemical reactions occur on the electrodes that. The battery releases a few electrons via a chemical. The electrons and ions flow because of the chemical reactions happening inside the battery—usually two of them going. The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. The flow of electrons provides an. It describes the method by which batteries generate electron flow as follows: However, there is a net flow of electrons since the ions include.
From www.bajeczneobrazy.pl
Electrolytic cell infographic diagram with components including anode Electrons In A Battery Flow From There's essentially no flow of individual free electrons inside the battery. One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. The electrons and ions flow because of the chemical reactions happening inside the battery—usually two of them going. When a. Electrons In A Battery Flow From.
From thekflow.blogspot.com
Vanadium redox flow batteries can provide cheap, largescale grid Electrons In A Battery Flow From The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. The battery releases a few electrons via a chemical. However,. Electrons In A Battery Flow From.
From stock.adobe.com
Dry cell. Cross section of battery with cathode, anode, paste, light Electrons In A Battery Flow From The electrons and ions flow because of the chemical reactions happening inside the battery—usually two of them going. The flow of electrons provides an. It describes the method by which batteries generate electron flow as follows: However, there is a net flow of electrons since the ions include. The battery releases a few electrons via a chemical. One electrode, known. Electrons In A Battery Flow From.
From somaap.org
What is the size of a battery wire, Battery Cable Charts Electrons In A Battery Flow From The battery releases a few electrons via a chemical. It describes the method by which batteries generate electron flow as follows: The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. The electrons and ions flow because of the chemical reactions happening inside the battery—usually two of them going.. Electrons In A Battery Flow From.
From somaap.org
What is the size of a battery wire, Battery Cable Charts Electrons In A Battery Flow From It describes the method by which batteries generate electron flow as follows: There's essentially no flow of individual free electrons inside the battery. When a device is connected to a battery — a light bulb or an electric circuit — chemical reactions occur on the electrodes that. One electrode, known as the cathode, connects to the positive end of the. Electrons In A Battery Flow From.
From cosmosmagazine.com
How do batteries work? Electrons In A Battery Flow From One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. There's essentially no flow of individual free electrons inside the battery. When a device is connected to a battery — a light bulb or an electric circuit — chemical reactions occur. Electrons In A Battery Flow From.
From www.agweb.com
Weird Science Which Way Does Electricity Flow Through a Vehicle’s Electrons In A Battery Flow From The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. The battery releases a few electrons via a chemical. However, there is a net flow of electrons since the ions include. When a device is connected to a battery — a light bulb or an electric circuit — chemical. Electrons In A Battery Flow From.
From arstechnica.com
Eternally five years away? No, batteries are improving under your nose Electrons In A Battery Flow From When a device is connected to a battery — a light bulb or an electric circuit — chemical reactions occur on the electrodes that. The electrons and ions flow because of the chemical reactions happening inside the battery—usually two of them going. One electrode, known as the cathode, connects to the positive end of the battery and is where the. Electrons In A Battery Flow From.
From www.alamy.com
Liion battery diagram. Vector illustration. Rechargeable battery in Electrons In A Battery Flow From When a device is connected to a battery — a light bulb or an electric circuit — chemical reactions occur on the electrodes that. The battery releases a few electrons via a chemical. The electrons and ions flow because of the chemical reactions happening inside the battery—usually two of them going. However, there is a net flow of electrons since. Electrons In A Battery Flow From.
From mybios.me
How Electrons Flow In A Battery Circuit Bios Pics Electrons In A Battery Flow From However, there is a net flow of electrons since the ions include. When a device is connected to a battery — a light bulb or an electric circuit — chemical reactions occur on the electrodes that. The electrons and ions flow because of the chemical reactions happening inside the battery—usually two of them going. There's essentially no flow of individual. Electrons In A Battery Flow From.
From www.slideserve.com
PPT Electrons flow from the __ terminal of the battery, through the Electrons In A Battery Flow From However, there is a net flow of electrons since the ions include. The flow of electrons provides an. There's essentially no flow of individual free electrons inside the battery. One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. The electrons. Electrons In A Battery Flow From.
From courses.lumenlearning.com
Galvanic Cells Chemistry Electrons In A Battery Flow From It describes the method by which batteries generate electron flow as follows: One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. The flow of electrons provides an. The electrons and ions flow because of the chemical reactions happening inside the. Electrons In A Battery Flow From.
From techcentral.co.za
Explainer How lithiumion batteries work TechCentral Electrons In A Battery Flow From However, there is a net flow of electrons since the ions include. The electrons and ions flow because of the chemical reactions happening inside the battery—usually two of them going. It describes the method by which batteries generate electron flow as follows: There's essentially no flow of individual free electrons inside the battery. One electrode, known as the cathode, connects. Electrons In A Battery Flow From.
From www.pinterest.com
Figure 1 In a battery, the flow of electrons goes from anode to Electrons In A Battery Flow From When a device is connected to a battery — a light bulb or an electric circuit — chemical reactions occur on the electrodes that. The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. The battery releases a few electrons via a chemical. One electrode, known as the cathode,. Electrons In A Battery Flow From.
From mx.nthu.edu.tw
main Electrons In A Battery Flow From However, there is a net flow of electrons since the ions include. When a device is connected to a battery — a light bulb or an electric circuit — chemical reactions occur on the electrodes that. The electrons and ions flow because of the chemical reactions happening inside the battery—usually two of them going. It describes the method by which. Electrons In A Battery Flow From.
From www.researchgate.net
Schematic illustration of a lithiumion battery (LIB) under discharge Electrons In A Battery Flow From There's essentially no flow of individual free electrons inside the battery. One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. When a device is connected to a battery — a light bulb or an electric circuit — chemical reactions occur. Electrons In A Battery Flow From.
From www.dreamstime.com
Electrons Flow on Cooper Wire, Learning Electricity Stock Illustration Electrons In A Battery Flow From It describes the method by which batteries generate electron flow as follows: The electrons and ions flow because of the chemical reactions happening inside the battery—usually two of them going. There's essentially no flow of individual free electrons inside the battery. The battery releases a few electrons via a chemical. One electrode, known as the cathode, connects to the positive. Electrons In A Battery Flow From.
From enginelibsaprozoic.z21.web.core.windows.net
Circuit Diagram Flow Of Electrons Electrons In A Battery Flow From It describes the method by which batteries generate electron flow as follows: The electrons and ions flow because of the chemical reactions happening inside the battery—usually two of them going. The battery releases a few electrons via a chemical. The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit.. Electrons In A Battery Flow From.
From energyknowledgebase.com
Electricity · Energy KnowledgeBase Electrons In A Battery Flow From One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. The battery releases a few electrons via a chemical. It describes the method by which batteries generate electron flow as follows: There's essentially no flow of individual free electrons inside the. Electrons In A Battery Flow From.
From marco-has-branch.blogspot.com
Which Statement Describes the Reactions in an Electrochemical Cell Electrons In A Battery Flow From It describes the method by which batteries generate electron flow as follows: The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. The battery releases a few electrons via a chemical. When a device is connected to a battery — a light bulb or an electric circuit — chemical. Electrons In A Battery Flow From.
From www.youtube.com
Two Battery Series Circuits Which Way Will Current Flow? (AP Physics Electrons In A Battery Flow From The electrons and ions flow because of the chemical reactions happening inside the battery—usually two of them going. However, there is a net flow of electrons since the ions include. The battery releases a few electrons via a chemical. One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves. Electrons In A Battery Flow From.
From in.pinterest.com
What is Electricity and Electric Current and its Units What is Electrons In A Battery Flow From The battery releases a few electrons via a chemical. However, there is a net flow of electrons since the ions include. The electrons and ions flow because of the chemical reactions happening inside the battery—usually two of them going. When a device is connected to a battery — a light bulb or an electric circuit — chemical reactions occur on. Electrons In A Battery Flow From.
From byjus.com
The conventional direction of electric current is the direction in Electrons In A Battery Flow From When a device is connected to a battery — a light bulb or an electric circuit — chemical reactions occur on the electrodes that. The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. However, there is a net flow of electrons since the ions include. There's essentially no. Electrons In A Battery Flow From.
From www.linuxfriends.net
Solar Energy Introduction Course Electrons In A Battery Flow From The electrons and ions flow because of the chemical reactions happening inside the battery—usually two of them going. One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. The chemical reactions in a battery involve the flow of electrons from one. Electrons In A Battery Flow From.
From socratic.org
Can you describe the process that releases electrons in a zinc copper Electrons In A Battery Flow From The flow of electrons provides an. However, there is a net flow of electrons since the ions include. One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. The electrons and ions flow because of the chemical reactions happening inside the. Electrons In A Battery Flow From.
From learn.sparkfun.com
What is Electricity? SparkFun Learn Electrons In A Battery Flow From It describes the method by which batteries generate electron flow as follows: However, there is a net flow of electrons since the ions include. The flow of electrons provides an. One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. There's. Electrons In A Battery Flow From.
From www.freepik.com
Premium Vector Electron flow and current flow Electric current Electrons In A Battery Flow From One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. The flow of electrons provides an. The electrons and ions flow because of the chemical reactions happening inside the battery—usually two of them going. The battery releases a few electrons via. Electrons In A Battery Flow From.
From www.slideserve.com
PPT Electricity PowerPoint Presentation, free download ID7008919 Electrons In A Battery Flow From The flow of electrons provides an. The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. There's essentially no flow of individual free electrons inside the battery. It describes the method by which batteries generate electron flow as follows: When a device is connected to a battery — a. Electrons In A Battery Flow From.
From enginewiringfrey.z21.web.core.windows.net
Electron Flow In Car Battery Electrons In A Battery Flow From There's essentially no flow of individual free electrons inside the battery. It describes the method by which batteries generate electron flow as follows: The electrons and ions flow because of the chemical reactions happening inside the battery—usually two of them going. One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical. Electrons In A Battery Flow From.
From schematica1qi3zl21.z21.web.core.windows.net
Current Flow In Circuit Diagram Electrons In A Battery Flow From One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. The electrons and ions flow because of the chemical reactions happening inside the battery—usually two of them going. The battery releases a few electrons via a chemical. The chemical reactions in. Electrons In A Battery Flow From.
From www.electricity-magnetism.org
Lithiumion Battery How it works Reaction, Anode & Cathode Electrons In A Battery Flow From When a device is connected to a battery — a light bulb or an electric circuit — chemical reactions occur on the electrodes that. The electrons and ions flow because of the chemical reactions happening inside the battery—usually two of them going. It describes the method by which batteries generate electron flow as follows: The battery releases a few electrons. Electrons In A Battery Flow From.
From www.linkedin.com
Understanding the Flow of Electrons in Charging LithiumIon Batteries Electrons In A Battery Flow From The flow of electrons provides an. It describes the method by which batteries generate electron flow as follows: One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. The chemical reactions in a battery involve the flow of electrons from one. Electrons In A Battery Flow From.
From energy.mit.edu
Flow batteries for gridscale energy storage MIT Energy Initiative Electrons In A Battery Flow From When a device is connected to a battery — a light bulb or an electric circuit — chemical reactions occur on the electrodes that. There's essentially no flow of individual free electrons inside the battery. The battery releases a few electrons via a chemical. It describes the method by which batteries generate electron flow as follows: The chemical reactions in. Electrons In A Battery Flow From.
From www.comsol.com
Does the Current Flow Backwards Inside a Battery? COMSOL Blog Electrons In A Battery Flow From The electrons and ions flow because of the chemical reactions happening inside the battery—usually two of them going. The battery releases a few electrons via a chemical. One electrode, known as the cathode, connects to the positive end of the battery and is where the electrical current leaves (or electrons enter) the battery during discharge, which. The flow of electrons. Electrons In A Battery Flow From.
From blog.upsbatterycenter.com
The Flow of Electrons Inside Battery Cells News about Energy Storage Electrons In A Battery Flow From When a device is connected to a battery — a light bulb or an electric circuit — chemical reactions occur on the electrodes that. The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. There's essentially no flow of individual free electrons inside the battery. One electrode, known as. Electrons In A Battery Flow From.