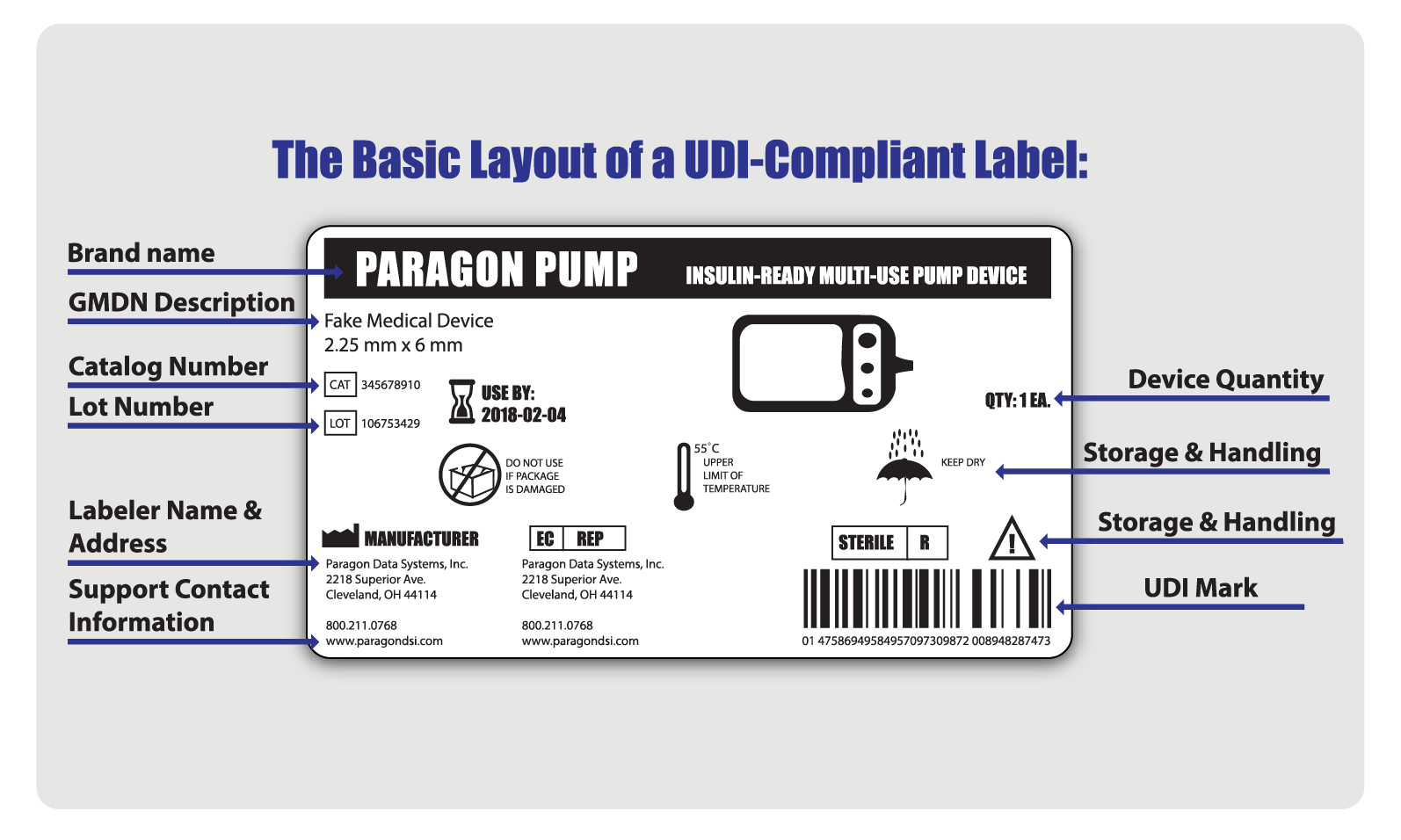
Over Labeling Medical Devices Fda . (a) a word, statement, or other information required. Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. Prominence of required label statements; Use of symbols in labeling. How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices?
from exogphupj.blob.core.windows.net
Prominence of required label statements; How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Use of symbols in labeling. (a) a word, statement, or other information required. Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over.
Medical Device Labelling Tga at William Maurer blog
Over Labeling Medical Devices Fda (a) a word, statement, or other information required. (a) a word, statement, or other information required. Prominence of required label statements; How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. Use of symbols in labeling.
From www.researchandmarkets.com
US FDA Labeling Requirements for Medical Devices Over Labeling Medical Devices Fda Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Use of symbols in labeling. Prominence of required label statements; (a) a word, statement, or other information required. Over Labeling Medical Devices Fda.
From knconsultingandservices.com
What is Labelling? Medical Device Consulting Company Over Labeling Medical Devices Fda How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? (a) a word, statement, or other information required. Use of symbols in labeling. Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. Prominence of required label statements; Over Labeling Medical Devices Fda.
From mungfali.com
Medical Device Labeling Symbols Over Labeling Medical Devices Fda Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. (a) a word, statement, or other information required. Prominence of required label statements; Use of symbols in labeling. How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Over Labeling Medical Devices Fda.
From blogs.sw.siemens.com
Siemens PLM for Medical Devices Labeling and UDI solution Over Labeling Medical Devices Fda Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Use of symbols in labeling. (a) a word, statement, or other information required. Prominence of required label statements; Over Labeling Medical Devices Fda.
From hxeskyyxe.blob.core.windows.net
Fda Medical Device Label Requirements at James Day blog Over Labeling Medical Devices Fda Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. (a) a word, statement, or other information required. Prominence of required label statements; Use of symbols in labeling. How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Over Labeling Medical Devices Fda.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Over Labeling Medical Devices Fda How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Prominence of required label statements; (a) a word, statement, or other information required. Use of symbols in labeling. Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. Over Labeling Medical Devices Fda.
From www.vrogue.co
Fda Medical Device Label Symbols vrogue.co Over Labeling Medical Devices Fda Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. Use of symbols in labeling. (a) a word, statement, or other information required. Prominence of required label statements; How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Over Labeling Medical Devices Fda.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Over Labeling Medical Devices Fda (a) a word, statement, or other information required. How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. Prominence of required label statements; Use of symbols in labeling. Over Labeling Medical Devices Fda.
From www.jdsupra.com
FDA Issues Final Rule on Use of Symbols in Labeling Knobbe Martens Over Labeling Medical Devices Fda (a) a word, statement, or other information required. Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. Use of symbols in labeling. How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Prominence of required label statements; Over Labeling Medical Devices Fda.
From giopglkhi.blob.core.windows.net
Health Canada Labeling Requirements at Julia Gillette blog Over Labeling Medical Devices Fda Use of symbols in labeling. Prominence of required label statements; Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. (a) a word, statement, or other information required. How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Over Labeling Medical Devices Fda.
From www.vrogue.co
Fda Medical Device Labeling Regulations Archives Medi vrogue.co Over Labeling Medical Devices Fda (a) a word, statement, or other information required. Prominence of required label statements; Use of symbols in labeling. How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. Over Labeling Medical Devices Fda.
From www.aplyon.com
Medical Device Labeling Procedure Bundle Over Labeling Medical Devices Fda Use of symbols in labeling. Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Prominence of required label statements; (a) a word, statement, or other information required. Over Labeling Medical Devices Fda.
From juleteagyd.blogspot.com
The Lot Number On A Medicine Label Identifies What Juleteagyd Over Labeling Medical Devices Fda Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. Use of symbols in labeling. How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? (a) a word, statement, or other information required. Prominence of required label statements; Over Labeling Medical Devices Fda.
From onlinelibrary.wiley.com
Do Healthcare Professionals Comprehend Standardized Symbols Present on Over Labeling Medical Devices Fda Prominence of required label statements; Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. Use of symbols in labeling. How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? (a) a word, statement, or other information required. Over Labeling Medical Devices Fda.
From www.vrogue.co
Fda Medical Device Labeling Requirements Presentation vrogue.co Over Labeling Medical Devices Fda How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Prominence of required label statements; (a) a word, statement, or other information required. Use of symbols in labeling. Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. Over Labeling Medical Devices Fda.
From vascufirst.com
What is the meaning of symbols on medical devices labels? VascuFirst Over Labeling Medical Devices Fda Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. Use of symbols in labeling. Prominence of required label statements; How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? (a) a word, statement, or other information required. Over Labeling Medical Devices Fda.
From exogmsrpv.blob.core.windows.net
Medical Device Labeling Font Size at Scott Bunger blog Over Labeling Medical Devices Fda Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. (a) a word, statement, or other information required. Use of symbols in labeling. Prominence of required label statements; How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Over Labeling Medical Devices Fda.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Over Labeling Medical Devices Fda How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Prominence of required label statements; Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. (a) a word, statement, or other information required. Use of symbols in labeling. Over Labeling Medical Devices Fda.
From www.fda.gov
The OvertheCounter Medicine Label Take a Look FDA Over Labeling Medical Devices Fda (a) a word, statement, or other information required. Use of symbols in labeling. Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. Prominence of required label statements; How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Over Labeling Medical Devices Fda.
From www.greenlight.guru
Am I Complying with FDA Medical Device Labeling Requirements? Over Labeling Medical Devices Fda How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. Use of symbols in labeling. Prominence of required label statements; (a) a word, statement, or other information required. Over Labeling Medical Devices Fda.
From www.vrogue.co
Fda Medical Device Label Symbols vrogue.co Over Labeling Medical Devices Fda Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. Prominence of required label statements; Use of symbols in labeling. (a) a word, statement, or other information required. How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Over Labeling Medical Devices Fda.
From www.pinterest.com
The FDA developed the new UniqueDeviceIdentifier (UDI) to improve the Over Labeling Medical Devices Fda Use of symbols in labeling. Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. Prominence of required label statements; (a) a word, statement, or other information required. How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Over Labeling Medical Devices Fda.
From andamanmed.com
Medical device labeling requirements in the Philippines Over Labeling Medical Devices Fda Prominence of required label statements; How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? (a) a word, statement, or other information required. Use of symbols in labeling. Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. Over Labeling Medical Devices Fda.
From vivafda.com
FDA Drug Labeling and Ingredient Requirement Viva FDA U.S. FDA Over Labeling Medical Devices Fda Prominence of required label statements; Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. (a) a word, statement, or other information required. Use of symbols in labeling. How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Over Labeling Medical Devices Fda.
From exovighly.blob.core.windows.net
Fda Medical Device Private Label Distributor at Jerry Robertson blog Over Labeling Medical Devices Fda Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. Prominence of required label statements; (a) a word, statement, or other information required. How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Use of symbols in labeling. Over Labeling Medical Devices Fda.
From old.sermitsiaq.ag
Medical Device Label Template Over Labeling Medical Devices Fda Prominence of required label statements; (a) a word, statement, or other information required. Use of symbols in labeling. How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. Over Labeling Medical Devices Fda.
From www.qualitymeddev.com
FDA Labelling Requirements for Medical Devices An Overview Over Labeling Medical Devices Fda Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Use of symbols in labeling. Prominence of required label statements; (a) a word, statement, or other information required. Over Labeling Medical Devices Fda.
From www.provisionfda.com
How to Determine Your Product’s FDA Category Is It a Cosmetic, Dietary Over Labeling Medical Devices Fda How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Prominence of required label statements; Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. Use of symbols in labeling. (a) a word, statement, or other information required. Over Labeling Medical Devices Fda.
From old.sermitsiaq.ag
Medical Device Label Template Over Labeling Medical Devices Fda Prominence of required label statements; Use of symbols in labeling. Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? (a) a word, statement, or other information required. Over Labeling Medical Devices Fda.
From alysidia.com
21 CFR Part 801 FDA Labeling Requirements for Medical Devices Over Labeling Medical Devices Fda (a) a word, statement, or other information required. Prominence of required label statements; Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Use of symbols in labeling. Over Labeling Medical Devices Fda.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Over Labeling Medical Devices Fda Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. (a) a word, statement, or other information required. Use of symbols in labeling. How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Prominence of required label statements; Over Labeling Medical Devices Fda.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Over Labeling Medical Devices Fda Use of symbols in labeling. Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. Prominence of required label statements; (a) a word, statement, or other information required. How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Over Labeling Medical Devices Fda.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Over Labeling Medical Devices Fda Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. Prominence of required label statements; How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Use of symbols in labeling. (a) a word, statement, or other information required. Over Labeling Medical Devices Fda.
From mungfali.com
FDA Medical Device Label Symbols Over Labeling Medical Devices Fda Prominence of required label statements; Use of symbols in labeling. (a) a word, statement, or other information required. How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. Over Labeling Medical Devices Fda.
From exogphupj.blob.core.windows.net
Medical Device Labelling Tga at William Maurer blog Over Labeling Medical Devices Fda Prominence of required label statements; Under the federal food, drug, and cosmetic act (fdca), fda has jurisdiction over medical device labeling [21 u.s.c.§352(a)] fda jurisdiction over. (a) a word, statement, or other information required. How do the regulatory authorities view overlabeling (i.e., placing a new label over another label) of medical devices? Use of symbols in labeling. Over Labeling Medical Devices Fda.