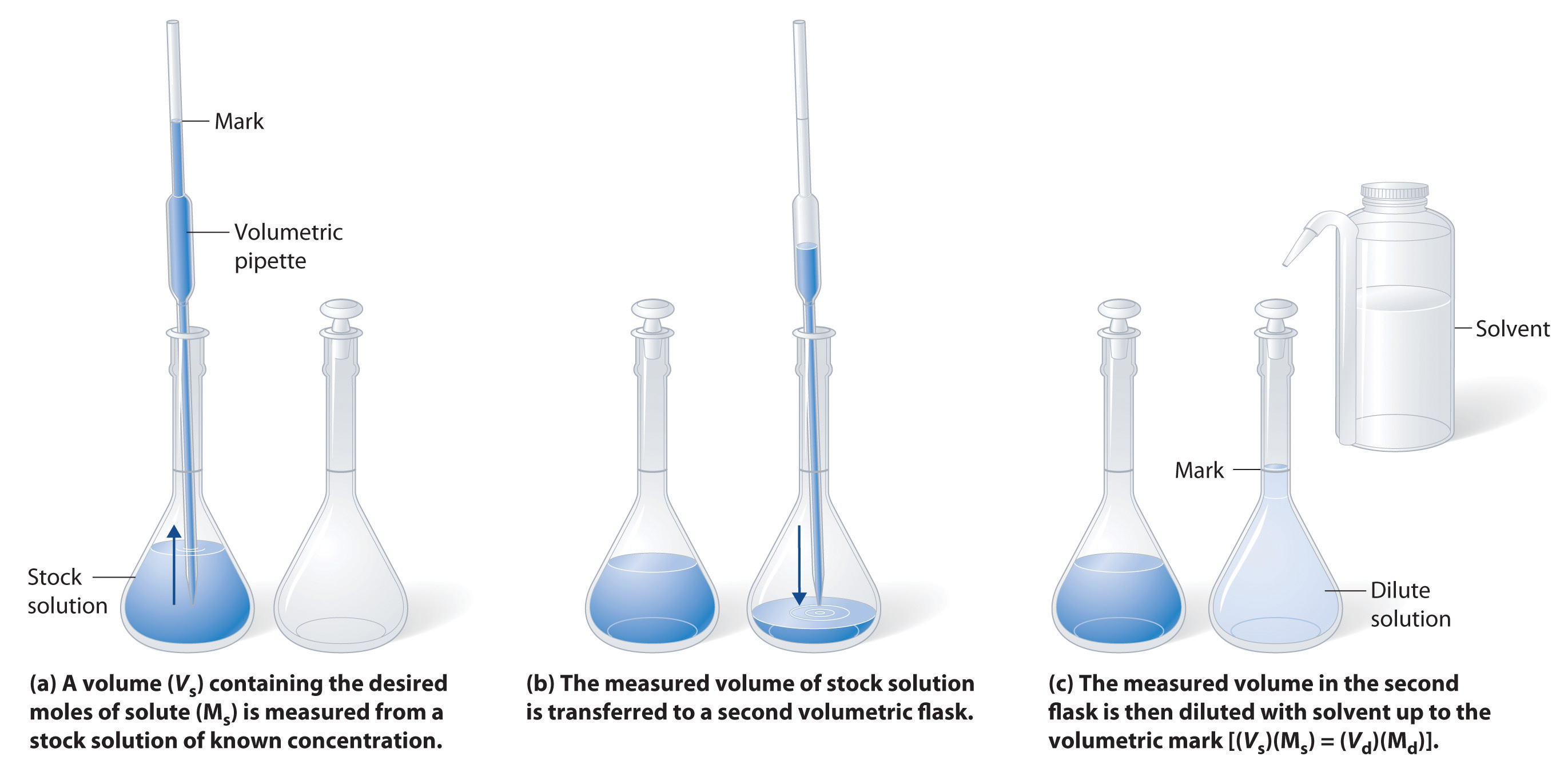
Dilution Titration Questions . Volumetric analysis is a process that uses the volume and concentration of one chemical reactant (standard solution) to determine the concentration of. Find the requested quantities in the following problems: A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. 10.0 ml of this solution is transferred to a. Use the information to determine the concentration of the hydrochloric acid. Deduce the number of moles. 1) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an. The concentration and the volumes change in a dilution. Moles of acid = concentration x volume in dm 3. Find the number of moles of acid.
from chem.libretexts.org
The concentration and the volumes change in a dilution. Use the information to determine the concentration of the hydrochloric acid. Moles of acid = concentration x volume in dm 3. Find the number of moles of acid. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. 1) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an. 10.0 ml of this solution is transferred to a. Volumetric analysis is a process that uses the volume and concentration of one chemical reactant (standard solution) to determine the concentration of. Deduce the number of moles. Find the requested quantities in the following problems:
5.2 Solutions and Dilutions Chemistry LibreTexts
Dilution Titration Questions 1) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an. Volumetric analysis is a process that uses the volume and concentration of one chemical reactant (standard solution) to determine the concentration of. Deduce the number of moles. 1) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an. Moles of acid = concentration x volume in dm 3. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. 10.0 ml of this solution is transferred to a. Use the information to determine the concentration of the hydrochloric acid. The concentration and the volumes change in a dilution. Find the number of moles of acid. Find the requested quantities in the following problems:
From www.youtube.com
Dilution Calculation Practice YouTube Dilution Titration Questions Use the information to determine the concentration of the hydrochloric acid. Find the number of moles of acid. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Moles of acid = concentration x volume in dm 3. The concentration and the volumes change in a dilution. Find the requested quantities. Dilution Titration Questions.
From lessonlibraryemersed.z13.web.core.windows.net
Titration Practical Questions And Answers Dilution Titration Questions Moles of acid = concentration x volume in dm 3. Find the number of moles of acid. Deduce the number of moles. Volumetric analysis is a process that uses the volume and concentration of one chemical reactant (standard solution) to determine the concentration of. The concentration and the volumes change in a dilution. A 1.0000 gram sample of k2co3 (138.2055. Dilution Titration Questions.
From www.youtube.com
A Level Chemistry Dilution Calculations Worked Example YouTube Dilution Titration Questions The concentration and the volumes change in a dilution. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Volumetric analysis is a process that uses the volume and concentration of one chemical reactant (standard solution) to determine the concentration of. Find the number of moles of acid. Find the requested. Dilution Titration Questions.
From edzion.com
HSC Titration Questions Edzion Dilution Titration Questions Use the information to determine the concentration of the hydrochloric acid. 1) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an. Find the number of moles of acid. 10.0 ml of this solution is transferred to a. Moles of acid = concentration x volume in dm 3. A 1.0000 gram sample of k2co3 (138.2055. Dilution Titration Questions.
From www.youtube.com
Video 18 Simple C1V1 = C2V2 dilution calculations YouTube Dilution Titration Questions 10.0 ml of this solution is transferred to a. Volumetric analysis is a process that uses the volume and concentration of one chemical reactant (standard solution) to determine the concentration of. Find the requested quantities in the following problems: Use the information to determine the concentration of the hydrochloric acid. The concentration and the volumes change in a dilution. A. Dilution Titration Questions.
From www.chegg.com
Titration results of 1/10 dilution. Dilution Titration Questions Deduce the number of moles. The concentration and the volumes change in a dilution. Moles of acid = concentration x volume in dm 3. Volumetric analysis is a process that uses the volume and concentration of one chemical reactant (standard solution) to determine the concentration of. 10.0 ml of this solution is transferred to a. A 1.0000 gram sample of. Dilution Titration Questions.
From www.chegg.com
Solved Answer the following questions regarding the dilution Dilution Titration Questions 1) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an. Moles of acid = concentration x volume in dm 3. The concentration and the volumes change in a dilution. Deduce the number of moles. 10.0 ml of this solution is transferred to a. Use the information to determine the concentration of the hydrochloric acid.. Dilution Titration Questions.
From www.youtube.com
Testing the validity of Ostwald’s dilution law/Determination of Dilution Titration Questions Find the number of moles of acid. 1) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an. Volumetric analysis is a process that uses the volume and concentration of one chemical reactant (standard solution) to determine the concentration of. Deduce the number of moles. Moles of acid = concentration x volume in dm 3.. Dilution Titration Questions.
From www.studocu.com
Dissolution Aqueous solutions, dilution, titration, practice problems Dilution Titration Questions Find the requested quantities in the following problems: Find the number of moles of acid. 1) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an. 10.0 ml of this solution is transferred to a. The concentration and the volumes change in a dilution. Use the information to determine the concentration of the hydrochloric acid.. Dilution Titration Questions.
From www.chegg.com
Solved Dilution diagrams can be very helpful in organizing Dilution Titration Questions Volumetric analysis is a process that uses the volume and concentration of one chemical reactant (standard solution) to determine the concentration of. Use the information to determine the concentration of the hydrochloric acid. The concentration and the volumes change in a dilution. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of. Dilution Titration Questions.
From www.youtube.com
Titration Calculations GCSE Science grade 7, 8 and 9 Booster Dilution Titration Questions Deduce the number of moles. The concentration and the volumes change in a dilution. Find the requested quantities in the following problems: Find the number of moles of acid. Use the information to determine the concentration of the hydrochloric acid. 10.0 ml of this solution is transferred to a. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in. Dilution Titration Questions.
From studylib.net
Steps in titration and serial dilution Dilution Titration Questions Moles of acid = concentration x volume in dm 3. Find the requested quantities in the following problems: 1) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an. Deduce the number of moles. 10.0 ml of this solution is transferred to a. The concentration and the volumes change in a dilution. Use the information. Dilution Titration Questions.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Dilution Titration Questions Moles of acid = concentration x volume in dm 3. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Find the requested quantities in the following problems: Deduce the number of moles. 1) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an. Volumetric analysis. Dilution Titration Questions.
From www.youtube.com
DilutionTitration Calculation ChemCram 101 Tutorial YouTube Dilution Titration Questions 10.0 ml of this solution is transferred to a. Volumetric analysis is a process that uses the volume and concentration of one chemical reactant (standard solution) to determine the concentration of. Find the requested quantities in the following problems: Use the information to determine the concentration of the hydrochloric acid. The concentration and the volumes change in a dilution. Find. Dilution Titration Questions.
From www.youtube.com
TITRATION EXAM QUESTION for A LEVEL CHEMISTRY YouTube Dilution Titration Questions Find the number of moles of acid. Moles of acid = concentration x volume in dm 3. The concentration and the volumes change in a dilution. Volumetric analysis is a process that uses the volume and concentration of one chemical reactant (standard solution) to determine the concentration of. Find the requested quantities in the following problems: Use the information to. Dilution Titration Questions.
From www.studypool.com
SOLUTION Titrations questions with answers Studypool Dilution Titration Questions Use the information to determine the concentration of the hydrochloric acid. The concentration and the volumes change in a dilution. 1) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an. Volumetric analysis is a process that uses the volume and concentration of one chemical reactant (standard solution) to determine the concentration of. 10.0 ml. Dilution Titration Questions.
From www.chegg.com
Solved To review how titration calculations work, here's a Dilution Titration Questions A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Use the information to determine the concentration of the hydrochloric acid. 1) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an. Find the requested quantities in the following problems: Find the number of moles of. Dilution Titration Questions.
From www.scribd.com
IGCSE Titration Practice Questions Dilution Titration Questions A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. The concentration and the volumes change in a dilution. 10.0 ml of this solution is transferred to a. Moles of acid = concentration x volume in dm 3. Volumetric analysis is a process that uses the volume and concentration of one. Dilution Titration Questions.
From www.numerade.com
SOLVED QUANTITATIVE ANALYSIS AcidBase Titrations with Volumetric Dilution Titration Questions Find the number of moles of acid. 10.0 ml of this solution is transferred to a. Find the requested quantities in the following problems: Deduce the number of moles. Moles of acid = concentration x volume in dm 3. The concentration and the volumes change in a dilution. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough. Dilution Titration Questions.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Dilution Titration Questions A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Volumetric analysis is a process that uses the volume and concentration of one chemical reactant (standard solution) to determine the concentration of. The concentration and the volumes change in a dilution. 10.0 ml of this solution is transferred to a. Use. Dilution Titration Questions.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilution Titration Questions Deduce the number of moles. The concentration and the volumes change in a dilution. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Use the information to determine the concentration of the hydrochloric acid. Volumetric analysis is a process that uses the volume and concentration of one chemical reactant (standard. Dilution Titration Questions.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution Titration Questions Volumetric analysis is a process that uses the volume and concentration of one chemical reactant (standard solution) to determine the concentration of. 1) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an. The concentration and the volumes change in a dilution. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water. Dilution Titration Questions.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Titration Questions 10.0 ml of this solution is transferred to a. Find the requested quantities in the following problems: Moles of acid = concentration x volume in dm 3. 1) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an. Volumetric analysis is a process that uses the volume and concentration of one chemical reactant (standard solution). Dilution Titration Questions.
From noel-kmclean.blogspot.com
Calculate Molarity of Acid in Titration Dilution Titration Questions Deduce the number of moles. 10.0 ml of this solution is transferred to a. The concentration and the volumes change in a dilution. Volumetric analysis is a process that uses the volume and concentration of one chemical reactant (standard solution) to determine the concentration of. 1) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of. Dilution Titration Questions.
From www.studocu.com
Dilution and Bacterial part 2 Questions (Answer all questions found Dilution Titration Questions Deduce the number of moles. Use the information to determine the concentration of the hydrochloric acid. The concentration and the volumes change in a dilution. 1) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution.. Dilution Titration Questions.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Dilution Titration Questions 1) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an. 10.0 ml of this solution is transferred to a. Find the number of moles of acid. The concentration and the volumes change in a dilution. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution.. Dilution Titration Questions.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilution Titration Questions The concentration and the volumes change in a dilution. 1) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an. Moles of acid = concentration x volume in dm 3. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Find the requested quantities in the. Dilution Titration Questions.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Dilution Titration Questions Deduce the number of moles. 10.0 ml of this solution is transferred to a. The concentration and the volumes change in a dilution. 1) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Moles of. Dilution Titration Questions.
From www.revisely.co.uk
ALevel AQA Chemistry Questions pH Curves, Titrations and Indicators Dilution Titration Questions Find the requested quantities in the following problems: Use the information to determine the concentration of the hydrochloric acid. The concentration and the volumes change in a dilution. 10.0 ml of this solution is transferred to a. 1) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an. Deduce the number of moles. Find the. Dilution Titration Questions.
From www.studypool.com
SOLUTION Igcse chem topic questions titration Studypool Dilution Titration Questions 10.0 ml of this solution is transferred to a. Use the information to determine the concentration of the hydrochloric acid. Find the number of moles of acid. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Deduce the number of moles. Find the requested quantities in the following problems: The. Dilution Titration Questions.
From www.numerade.com
SOLVED Ex.3 Data Sheet Using dilution Eactor in an Acid Base Titration Dilution Titration Questions Volumetric analysis is a process that uses the volume and concentration of one chemical reactant (standard solution) to determine the concentration of. Use the information to determine the concentration of the hydrochloric acid. 1) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an. Find the number of moles of acid. 10.0 ml of this. Dilution Titration Questions.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Dilution Titration Questions A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. 1) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an. Find the requested quantities in the following problems: Use the information to determine the concentration of the hydrochloric acid. Moles of acid = concentration x. Dilution Titration Questions.
From wiener.me
Dilution Techniques And Calculations, 44 OFF wiener.me Dilution Titration Questions Find the number of moles of acid. Volumetric analysis is a process that uses the volume and concentration of one chemical reactant (standard solution) to determine the concentration of. 1) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make. Dilution Titration Questions.
From www.youtube.com
Widal test slide titration/dilution method.Procedure and result Dilution Titration Questions The concentration and the volumes change in a dilution. Volumetric analysis is a process that uses the volume and concentration of one chemical reactant (standard solution) to determine the concentration of. Find the number of moles of acid. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Find the requested. Dilution Titration Questions.
From www.youtube.com
Chemistry Concepts Dilution and Titration Calculations.mp4 YouTube Dilution Titration Questions 1) if it takes 54 ml of 0.1 m naoh to neutralize 125 ml of an. Use the information to determine the concentration of the hydrochloric acid. Deduce the number of moles. Find the number of moles of acid. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. The concentration. Dilution Titration Questions.