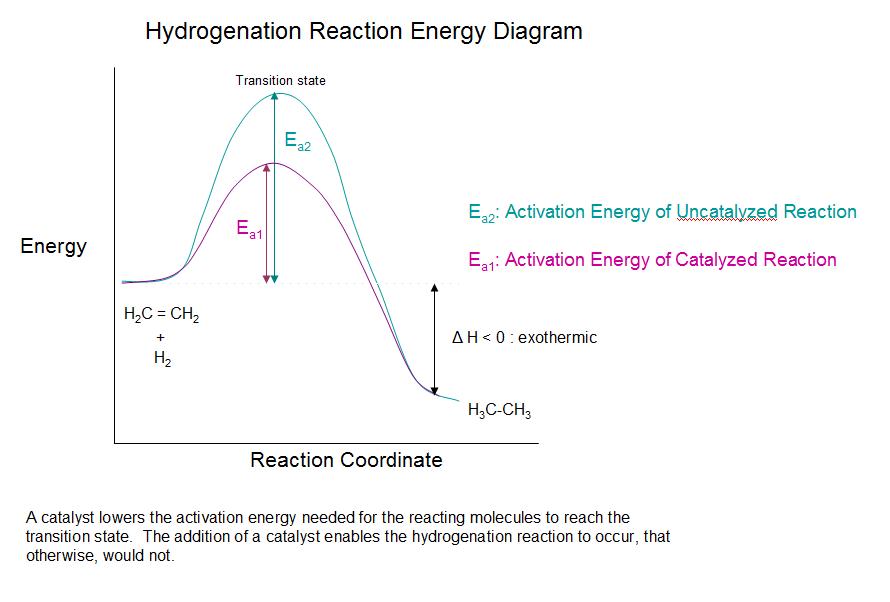
Activation Energy Diagram With Catalyst . To increase the rate of a reaction you need to increase the number of successful collisions. The catalyst provides a different reaction path with a lower. One without a catalyst and one with a catalyst. catalysts and activation energy. this potential energy diagram shows the effect of a catalyst on the activation energy. One possible way of doing this. reaction diagrams for a chemical process with and without a catalyst are shown below. the two reaction diagrams here represent the same reaction: in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the. One without a catalyst and one with a catalyst. the two reaction diagrams here represent the same reaction: this potential energy diagram shows the effect of a catalyst on the activation energy.
from chem.libretexts.org
in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the. One possible way of doing this. the two reaction diagrams here represent the same reaction: reaction diagrams for a chemical process with and without a catalyst are shown below. The catalyst provides a different reaction path with a lower. To increase the rate of a reaction you need to increase the number of successful collisions. the two reaction diagrams here represent the same reaction: catalysts and activation energy. One without a catalyst and one with a catalyst. One without a catalyst and one with a catalyst.
Catalytic Hydrogenation of Alkenes Chemistry LibreTexts
Activation Energy Diagram With Catalyst One without a catalyst and one with a catalyst. The catalyst provides a different reaction path with a lower. One without a catalyst and one with a catalyst. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the. the two reaction diagrams here represent the same reaction: this potential energy diagram shows the effect of a catalyst on the activation energy. catalysts and activation energy. One possible way of doing this. To increase the rate of a reaction you need to increase the number of successful collisions. the two reaction diagrams here represent the same reaction: One without a catalyst and one with a catalyst. this potential energy diagram shows the effect of a catalyst on the activation energy. reaction diagrams for a chemical process with and without a catalyst are shown below.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Activation Energy Diagram With Catalyst catalysts and activation energy. the two reaction diagrams here represent the same reaction: One without a catalyst and one with a catalyst. One possible way of doing this. One without a catalyst and one with a catalyst. The catalyst provides a different reaction path with a lower. To increase the rate of a reaction you need to increase. Activation Energy Diagram With Catalyst.
From courses.lumenlearning.com
12.7 Catalysis General College Chemistry II Activation Energy Diagram With Catalyst catalysts and activation energy. this potential energy diagram shows the effect of a catalyst on the activation energy. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the. One without a catalyst and one with a catalyst. One possible way of doing this. To increase the rate of. Activation Energy Diagram With Catalyst.
From www.studyorgo.com
Energy Diagram Module Series Part Three Intermediates and Rate Activation Energy Diagram With Catalyst in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the. this potential energy diagram shows the effect of a catalyst on the activation energy. reaction diagrams for a chemical process with and without a catalyst are shown below. One without a catalyst and one with a catalyst. To. Activation Energy Diagram With Catalyst.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Activation Energy Diagram With Catalyst One possible way of doing this. To increase the rate of a reaction you need to increase the number of successful collisions. this potential energy diagram shows the effect of a catalyst on the activation energy. the two reaction diagrams here represent the same reaction: The catalyst provides a different reaction path with a lower. reaction diagrams. Activation Energy Diagram With Catalyst.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Activation Energy Diagram With Catalyst One possible way of doing this. the two reaction diagrams here represent the same reaction: this potential energy diagram shows the effect of a catalyst on the activation energy. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the. To increase the rate of a reaction you need. Activation Energy Diagram With Catalyst.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Activation Energy Diagram With Catalyst this potential energy diagram shows the effect of a catalyst on the activation energy. One possible way of doing this. the two reaction diagrams here represent the same reaction: catalysts and activation energy. this potential energy diagram shows the effect of a catalyst on the activation energy. in the case of a biological reaction, when. Activation Energy Diagram With Catalyst.
From iconpasob.blogg.se
iconpasob.blogg.se How much does catalyst need of activation energy Activation Energy Diagram With Catalyst this potential energy diagram shows the effect of a catalyst on the activation energy. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the. To increase the rate of a reaction you need to increase the number of successful collisions. One without a catalyst and one with a catalyst.. Activation Energy Diagram With Catalyst.
From large.stanford.edu
Catalysts in 21st Century Energy Activation Energy Diagram With Catalyst in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the. catalysts and activation energy. The catalyst provides a different reaction path with a lower. One without a catalyst and one with a catalyst. the two reaction diagrams here represent the same reaction: this potential energy diagram shows. Activation Energy Diagram With Catalyst.
From www.chim.lu
Activation energy and catalysis Activation Energy Diagram With Catalyst catalysts and activation energy. the two reaction diagrams here represent the same reaction: To increase the rate of a reaction you need to increase the number of successful collisions. reaction diagrams for a chemical process with and without a catalyst are shown below. in the case of a biological reaction, when an enzyme (a form of. Activation Energy Diagram With Catalyst.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog Activation Energy Diagram With Catalyst this potential energy diagram shows the effect of a catalyst on the activation energy. reaction diagrams for a chemical process with and without a catalyst are shown below. One without a catalyst and one with a catalyst. One without a catalyst and one with a catalyst. The catalyst provides a different reaction path with a lower. this. Activation Energy Diagram With Catalyst.
From schoolbag.info
A catalyst speeds up a reaction by providing the reactants with an Activation Energy Diagram With Catalyst in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the. the two reaction diagrams here represent the same reaction: The catalyst provides a different reaction path with a lower. One without a catalyst and one with a catalyst. reaction diagrams for a chemical process with and without a. Activation Energy Diagram With Catalyst.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Activation Energy Diagram With Catalyst this potential energy diagram shows the effect of a catalyst on the activation energy. catalysts and activation energy. One without a catalyst and one with a catalyst. To increase the rate of a reaction you need to increase the number of successful collisions. this potential energy diagram shows the effect of a catalyst on the activation energy.. Activation Energy Diagram With Catalyst.
From psu.pb.unizin.org
12.7 Catalysis Chemistry 112 Chapters 1217 of OpenStax General Activation Energy Diagram With Catalyst The catalyst provides a different reaction path with a lower. One possible way of doing this. the two reaction diagrams here represent the same reaction: One without a catalyst and one with a catalyst. reaction diagrams for a chemical process with and without a catalyst are shown below. the two reaction diagrams here represent the same reaction:. Activation Energy Diagram With Catalyst.
From guidepartrefractor.z21.web.core.windows.net
Energy Diagram Catalyzed Reaction Activation Energy Diagram With Catalyst catalysts and activation energy. this potential energy diagram shows the effect of a catalyst on the activation energy. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the. reaction diagrams for a chemical process with and without a catalyst are shown below. the two reaction diagrams. Activation Energy Diagram With Catalyst.
From telegra.ph
Catalyst activation energy diagram of enzymes Telegraph Activation Energy Diagram With Catalyst the two reaction diagrams here represent the same reaction: The catalyst provides a different reaction path with a lower. reaction diagrams for a chemical process with and without a catalyst are shown below. catalysts and activation energy. One without a catalyst and one with a catalyst. the two reaction diagrams here represent the same reaction: . Activation Energy Diagram With Catalyst.
From www.researchgate.net
Free energy of activation of uncatalyzed and catalyzed reactions Activation Energy Diagram With Catalyst One without a catalyst and one with a catalyst. reaction diagrams for a chemical process with and without a catalyst are shown below. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the. One without a catalyst and one with a catalyst. the two reaction diagrams here represent. Activation Energy Diagram With Catalyst.
From www.transtutors.com
(Solved) The diagram shown above shows the reaction profile for the Activation Energy Diagram With Catalyst the two reaction diagrams here represent the same reaction: To increase the rate of a reaction you need to increase the number of successful collisions. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the. catalysts and activation energy. The catalyst provides a different reaction path with a. Activation Energy Diagram With Catalyst.
From lavelle.chem.ucla.edu
Catalyst effect on energy diagram CHEMISTRY COMMUNITY Activation Energy Diagram With Catalyst in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the. reaction diagrams for a chemical process with and without a catalyst are shown below. the two reaction diagrams here represent the same reaction: To increase the rate of a reaction you need to increase the number of successful. Activation Energy Diagram With Catalyst.
From dragonbeeotch4sschematic.z21.web.core.windows.net
Energy Diagram For Chemical Reaction Activation Energy Diagram With Catalyst catalysts and activation energy. To increase the rate of a reaction you need to increase the number of successful collisions. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the. The catalyst provides a different reaction path with a lower. this potential energy diagram shows the effect of. Activation Energy Diagram With Catalyst.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Activation Energy Diagram With Catalyst the two reaction diagrams here represent the same reaction: reaction diagrams for a chemical process with and without a catalyst are shown below. One without a catalyst and one with a catalyst. One without a catalyst and one with a catalyst. this potential energy diagram shows the effect of a catalyst on the activation energy. in. Activation Energy Diagram With Catalyst.
From ar.inspiredpencil.com
Activation Energy Diagram Activation Energy Diagram With Catalyst One without a catalyst and one with a catalyst. One possible way of doing this. catalysts and activation energy. the two reaction diagrams here represent the same reaction: reaction diagrams for a chemical process with and without a catalyst are shown below. this potential energy diagram shows the effect of a catalyst on the activation energy.. Activation Energy Diagram With Catalyst.
From www.expii.com
Energy Diagram — Overview & Parts Expii Activation Energy Diagram With Catalyst in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the. One without a catalyst and one with a catalyst. the two reaction diagrams here represent the same reaction: One possible way of doing this. catalysts and activation energy. this potential energy diagram shows the effect of a. Activation Energy Diagram With Catalyst.
From chem.libretexts.org
Catalytic Hydrogenation of Alkenes Chemistry LibreTexts Activation Energy Diagram With Catalyst catalysts and activation energy. this potential energy diagram shows the effect of a catalyst on the activation energy. the two reaction diagrams here represent the same reaction: One without a catalyst and one with a catalyst. this potential energy diagram shows the effect of a catalyst on the activation energy. One without a catalyst and one. Activation Energy Diagram With Catalyst.
From byjus.com
Reaction Coordinate Diagram An Overview of Reaction Coordinate Activation Energy Diagram With Catalyst The catalyst provides a different reaction path with a lower. the two reaction diagrams here represent the same reaction: this potential energy diagram shows the effect of a catalyst on the activation energy. One without a catalyst and one with a catalyst. the two reaction diagrams here represent the same reaction: One possible way of doing this.. Activation Energy Diagram With Catalyst.
From www.workmanagementsolutions.com.au
An Analogy The Activation Energy and Catalysis in Business Operations Activation Energy Diagram With Catalyst The catalyst provides a different reaction path with a lower. To increase the rate of a reaction you need to increase the number of successful collisions. One possible way of doing this. the two reaction diagrams here represent the same reaction: this potential energy diagram shows the effect of a catalyst on the activation energy. catalysts and. Activation Energy Diagram With Catalyst.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.9.3 MaxwellBoltzmann Distributions翰林国际教育 Activation Energy Diagram With Catalyst To increase the rate of a reaction you need to increase the number of successful collisions. this potential energy diagram shows the effect of a catalyst on the activation energy. reaction diagrams for a chemical process with and without a catalyst are shown below. One without a catalyst and one with a catalyst. catalysts and activation energy.. Activation Energy Diagram With Catalyst.
From www.doubtnut.com
X = energy of activation with catalyst, Y = energy of activation wit Activation Energy Diagram With Catalyst this potential energy diagram shows the effect of a catalyst on the activation energy. this potential energy diagram shows the effect of a catalyst on the activation energy. To increase the rate of a reaction you need to increase the number of successful collisions. the two reaction diagrams here represent the same reaction: One possible way of. Activation Energy Diagram With Catalyst.
From www.cheric.org
Chemical Reaction (Reaction rate) Activation Energy Diagram With Catalyst this potential energy diagram shows the effect of a catalyst on the activation energy. reaction diagrams for a chemical process with and without a catalyst are shown below. One possible way of doing this. the two reaction diagrams here represent the same reaction: To increase the rate of a reaction you need to increase the number of. Activation Energy Diagram With Catalyst.
From dragonbeeotch4sschematic.z21.web.core.windows.net
Energy Diagram Activation Energy Activation Energy Diagram With Catalyst One without a catalyst and one with a catalyst. this potential energy diagram shows the effect of a catalyst on the activation energy. this potential energy diagram shows the effect of a catalyst on the activation energy. catalysts and activation energy. One without a catalyst and one with a catalyst. reaction diagrams for a chemical process. Activation Energy Diagram With Catalyst.
From infinitylearn.com
A catalyst increases the rate of reaction by Sri Chaitanya Infinity Activation Energy Diagram With Catalyst catalysts and activation energy. One without a catalyst and one with a catalyst. The catalyst provides a different reaction path with a lower. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the. reaction diagrams for a chemical process with and without a catalyst are shown below. One. Activation Energy Diagram With Catalyst.
From cheminfo.uz
Kataliz va katalizator haqida ChemInfo.uz Activation Energy Diagram With Catalyst the two reaction diagrams here represent the same reaction: One without a catalyst and one with a catalyst. this potential energy diagram shows the effect of a catalyst on the activation energy. reaction diagrams for a chemical process with and without a catalyst are shown below. One possible way of doing this. the two reaction diagrams. Activation Energy Diagram With Catalyst.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Activation Energy Diagram With Catalyst reaction diagrams for a chemical process with and without a catalyst are shown below. the two reaction diagrams here represent the same reaction: the two reaction diagrams here represent the same reaction: catalysts and activation energy. To increase the rate of a reaction you need to increase the number of successful collisions. One possible way of. Activation Energy Diagram With Catalyst.
From 2012books.lardbucket.org
Catalysis Activation Energy Diagram With Catalyst One possible way of doing this. To increase the rate of a reaction you need to increase the number of successful collisions. One without a catalyst and one with a catalyst. this potential energy diagram shows the effect of a catalyst on the activation energy. the two reaction diagrams here represent the same reaction: One without a catalyst. Activation Energy Diagram With Catalyst.
From as-bio-and-chem.blogspot.com
Bio+Chem Notes. ^^ Recapping Rates of Reaction Activation Energy Diagram With Catalyst catalysts and activation energy. this potential energy diagram shows the effect of a catalyst on the activation energy. the two reaction diagrams here represent the same reaction: this potential energy diagram shows the effect of a catalyst on the activation energy. One without a catalyst and one with a catalyst. The catalyst provides a different reaction. Activation Energy Diagram With Catalyst.
From proper-cooking.info
Activation Energy Graph With Catalyst Activation Energy Diagram With Catalyst the two reaction diagrams here represent the same reaction: this potential energy diagram shows the effect of a catalyst on the activation energy. catalysts and activation energy. in the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the. One without a catalyst and one with a catalyst. One. Activation Energy Diagram With Catalyst.