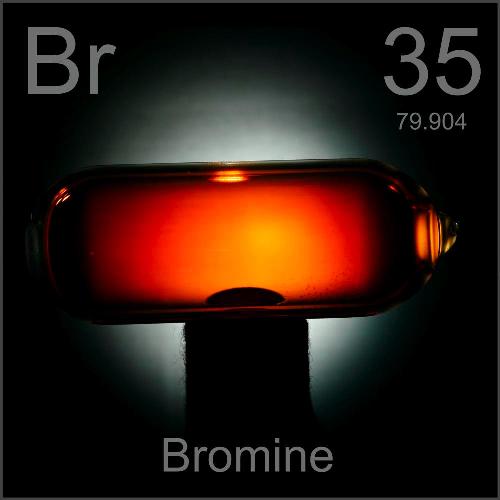
Bromine Trichloride Color . Let us follow a series of steps that will ease our understanding of the methodology to draw lewis structure of brcl3: for brcl3, the lewis structure reveals that a total of 28 valence electrons(7 from bromine + 7(3) from 3chlorine atoms) are required to form a single brcl3 molecule. bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at 200å c. It is physically stable, but reacts violently with water and most metals and nonmetals. bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at. thallium tribromide can be prepared in ch 3 cn by treating a solution of the monobromide with bromine gas,.
from www.myinterestingfacts.com
It is physically stable, but reacts violently with water and most metals and nonmetals. bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at 200å c. bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at. thallium tribromide can be prepared in ch 3 cn by treating a solution of the monobromide with bromine gas,. for brcl3, the lewis structure reveals that a total of 28 valence electrons(7 from bromine + 7(3) from 3chlorine atoms) are required to form a single brcl3 molecule. Let us follow a series of steps that will ease our understanding of the methodology to draw lewis structure of brcl3:
10 Interesting the Element Bromine Facts My Interesting Facts
Bromine Trichloride Color bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at. thallium tribromide can be prepared in ch 3 cn by treating a solution of the monobromide with bromine gas,. It is physically stable, but reacts violently with water and most metals and nonmetals. Let us follow a series of steps that will ease our understanding of the methodology to draw lewis structure of brcl3: for brcl3, the lewis structure reveals that a total of 28 valence electrons(7 from bromine + 7(3) from 3chlorine atoms) are required to form a single brcl3 molecule. bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at. bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at 200å c.
From joelgordon.photoshelter.com
Chlorine Bromine Iodine Joel Gordon Photography Bromine Trichloride Color for brcl3, the lewis structure reveals that a total of 28 valence electrons(7 from bromine + 7(3) from 3chlorine atoms) are required to form a single brcl3 molecule. thallium tribromide can be prepared in ch 3 cn by treating a solution of the monobromide with bromine gas,. It is physically stable, but reacts violently with water and most. Bromine Trichloride Color.
From images-of-elements.com
Chemical Elements Bromine Bromine Trichloride Color bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at. thallium tribromide can be prepared in ch 3 cn by treating a solution of the monobromide with bromine gas,. for brcl3, the lewis structure reveals that a total of 28 valence electrons(7 from bromine + 7(3) from 3chlorine atoms) are. Bromine Trichloride Color.
From www.chemistrylearner.com
Bromine Facts, Symbol, Discovery, Properties, Uses Bromine Trichloride Color Let us follow a series of steps that will ease our understanding of the methodology to draw lewis structure of brcl3: thallium tribromide can be prepared in ch 3 cn by treating a solution of the monobromide with bromine gas,. for brcl3, the lewis structure reveals that a total of 28 valence electrons(7 from bromine + 7(3) from. Bromine Trichloride Color.
From www.indiamart.com
Liquid Bromine, 7726956, तरल ब्रोमिन Reliable Chemicals, Ulhasnagar Bromine Trichloride Color Let us follow a series of steps that will ease our understanding of the methodology to draw lewis structure of brcl3: It is physically stable, but reacts violently with water and most metals and nonmetals. for brcl3, the lewis structure reveals that a total of 28 valence electrons(7 from bromine + 7(3) from 3chlorine atoms) are required to form. Bromine Trichloride Color.
From www.coursehero.com
[Solved] Chemical beryllium nitrogen bromine Part B chloride bromine Bromine Trichloride Color bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at. It is physically stable, but reacts violently with water and most metals and nonmetals. Let us follow a series of steps that will ease our understanding of the methodology to draw lewis structure of brcl3: thallium tribromide can be prepared in. Bromine Trichloride Color.
From www.bbc.com
Who's afraid of bromine? BBC News Bromine Trichloride Color bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at. bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at 200å c. It is physically stable, but reacts violently with water and most metals and nonmetals. for brcl3, the lewis structure reveals that. Bromine Trichloride Color.
From www.pngwing.com
Halide Chlorobenzene Bromobenzene Chloride Bromine, others, angle Bromine Trichloride Color Let us follow a series of steps that will ease our understanding of the methodology to draw lewis structure of brcl3: thallium tribromide can be prepared in ch 3 cn by treating a solution of the monobromide with bromine gas,. bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at 200å. Bromine Trichloride Color.
From www.dreamstime.com
BrCl3 Bromine Trichloride CAS Chemical Substance in White Plastic Bromine Trichloride Color thallium tribromide can be prepared in ch 3 cn by treating a solution of the monobromide with bromine gas,. Let us follow a series of steps that will ease our understanding of the methodology to draw lewis structure of brcl3: bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at. . Bromine Trichloride Color.
From www.dreamstime.com
Bromine Chemical Stock Photos Free & RoyaltyFree Stock Photos from Bromine Trichloride Color Let us follow a series of steps that will ease our understanding of the methodology to draw lewis structure of brcl3: thallium tribromide can be prepared in ch 3 cn by treating a solution of the monobromide with bromine gas,. It is physically stable, but reacts violently with water and most metals and nonmetals. bromine pentafluoride (brf 5). Bromine Trichloride Color.
From www.dreamstime.com
Bromine Chemistry Element Symbol Icon. Chemical Education Science Atom Bromine Trichloride Color It is physically stable, but reacts violently with water and most metals and nonmetals. for brcl3, the lewis structure reveals that a total of 28 valence electrons(7 from bromine + 7(3) from 3chlorine atoms) are required to form a single brcl3 molecule. bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine. Bromine Trichloride Color.
From fphoto.photoshelter.com
science element bromine Fundamental Photographs The Art of Science Bromine Trichloride Color thallium tribromide can be prepared in ch 3 cn by treating a solution of the monobromide with bromine gas,. Let us follow a series of steps that will ease our understanding of the methodology to draw lewis structure of brcl3: bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at. It. Bromine Trichloride Color.
From www.inforad.co.kr
화합물 사전 브로민(Bromine) Bromine Trichloride Color bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at. bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at 200å c. for brcl3, the lewis structure reveals that a total of 28 valence electrons(7 from bromine + 7(3) from 3chlorine atoms) are. Bromine Trichloride Color.
From images-of-elements.com
Chemical Elements Bromine Bromine Trichloride Color bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at 200å c. for brcl3, the lewis structure reveals that a total of 28 valence electrons(7 from bromine + 7(3) from 3chlorine atoms) are required to form a single brcl3 molecule. Let us follow a series of steps that will ease our. Bromine Trichloride Color.
From fphoto.photoshelter.com
science chemistry reaction bromine carbon tetrachloride Fundamental Bromine Trichloride Color Let us follow a series of steps that will ease our understanding of the methodology to draw lewis structure of brcl3: bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at. It is physically stable, but reacts violently with water and most metals and nonmetals. thallium tribromide can be prepared in. Bromine Trichloride Color.
From fphoto.photoshelter.com
science element bromine halogen Fundamental Photographs The Art of Bromine Trichloride Color It is physically stable, but reacts violently with water and most metals and nonmetals. thallium tribromide can be prepared in ch 3 cn by treating a solution of the monobromide with bromine gas,. bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at 200å c. Let us follow a series of. Bromine Trichloride Color.
From www.youtube.com
Bromine Element Series YouTube Bromine Trichloride Color thallium tribromide can be prepared in ch 3 cn by treating a solution of the monobromide with bromine gas,. bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at 200å c. for brcl3, the lewis structure reveals that a total of 28 valence electrons(7 from bromine + 7(3) from 3chlorine. Bromine Trichloride Color.
From www.myinterestingfacts.com
10 Interesting the Element Bromine Facts My Interesting Facts Bromine Trichloride Color Let us follow a series of steps that will ease our understanding of the methodology to draw lewis structure of brcl3: It is physically stable, but reacts violently with water and most metals and nonmetals. thallium tribromide can be prepared in ch 3 cn by treating a solution of the monobromide with bromine gas,. for brcl3, the lewis. Bromine Trichloride Color.
From www.youtube.com
Is BrCl3 Polar or Nonpolar (Bromine Trichloride) YouTube Bromine Trichloride Color bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at. thallium tribromide can be prepared in ch 3 cn by treating a solution of the monobromide with bromine gas,. It is physically stable, but reacts violently with water and most metals and nonmetals. for brcl3, the lewis structure reveals that. Bromine Trichloride Color.
From alannameowarias.blogspot.com
Colour of Bromine Water Bromine Trichloride Color It is physically stable, but reacts violently with water and most metals and nonmetals. thallium tribromide can be prepared in ch 3 cn by treating a solution of the monobromide with bromine gas,. for brcl3, the lewis structure reveals that a total of 28 valence electrons(7 from bromine + 7(3) from 3chlorine atoms) are required to form a. Bromine Trichloride Color.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds Bromine Trichloride Color bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at. thallium tribromide can be prepared in ch 3 cn by treating a solution of the monobromide with bromine gas,. Let us follow a series of steps that will ease our understanding of the methodology to draw lewis structure of brcl3: It. Bromine Trichloride Color.
From theodoregray.com
Facts, pictures, stories about the element Bromine in the Periodic Table Bromine Trichloride Color thallium tribromide can be prepared in ch 3 cn by treating a solution of the monobromide with bromine gas,. Let us follow a series of steps that will ease our understanding of the methodology to draw lewis structure of brcl3: bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at. . Bromine Trichloride Color.
From www.youtube.com
Bromine Water + Sodium Chloride YouTube Bromine Trichloride Color bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at 200å c. for brcl3, the lewis structure reveals that a total of 28 valence electrons(7 from bromine + 7(3) from 3chlorine atoms) are required to form a single brcl3 molecule. bromine pentafluoride (brf 5) is a colorless fuming liquid, made. Bromine Trichloride Color.
From www.flaticon.com
Bromine Free education icons Bromine Trichloride Color bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at. for brcl3, the lewis structure reveals that a total of 28 valence electrons(7 from bromine + 7(3) from 3chlorine atoms) are required to form a single brcl3 molecule. It is physically stable, but reacts violently with water and most metals and. Bromine Trichloride Color.
From studiousguy.com
Bromine (Br) Properties & Uses StudiousGuy Bromine Trichloride Color Let us follow a series of steps that will ease our understanding of the methodology to draw lewis structure of brcl3: bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at 200å c. bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at. . Bromine Trichloride Color.
From www.transtutors.com
(Solved) ) 8) Bromine trichloride BrCl3 Lewis structure Electron pair Bromine Trichloride Color bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at. for brcl3, the lewis structure reveals that a total of 28 valence electrons(7 from bromine + 7(3) from 3chlorine atoms) are required to form a single brcl3 molecule. It is physically stable, but reacts violently with water and most metals and. Bromine Trichloride Color.
From www.freepik.com
Premium Vector Chemical element bromine vector illustration Bromine Trichloride Color thallium tribromide can be prepared in ch 3 cn by treating a solution of the monobromide with bromine gas,. It is physically stable, but reacts violently with water and most metals and nonmetals. Let us follow a series of steps that will ease our understanding of the methodology to draw lewis structure of brcl3: bromine pentafluoride (brf 5). Bromine Trichloride Color.
From www.alamy.com
Bromine. Bromum. Halogens. Chemical Element of Mendeleev's Periodic Bromine Trichloride Color Let us follow a series of steps that will ease our understanding of the methodology to draw lewis structure of brcl3: thallium tribromide can be prepared in ch 3 cn by treating a solution of the monobromide with bromine gas,. It is physically stable, but reacts violently with water and most metals and nonmetals. bromine pentafluoride (brf 5). Bromine Trichloride Color.
From www.numerade.com
SOLVED Draw the Lewis structure of bromine trichloride (BrCl3) Marvin Bromine Trichloride Color Let us follow a series of steps that will ease our understanding of the methodology to draw lewis structure of brcl3: bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at. bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at 200å c. It. Bromine Trichloride Color.
From theodoregray.com
Facts, pictures, stories about the element Bromine in the Periodic Table Bromine Trichloride Color It is physically stable, but reacts violently with water and most metals and nonmetals. for brcl3, the lewis structure reveals that a total of 28 valence electrons(7 from bromine + 7(3) from 3chlorine atoms) are required to form a single brcl3 molecule. Let us follow a series of steps that will ease our understanding of the methodology to draw. Bromine Trichloride Color.
From images-of-elements.com
Chemical Elements Bromine Bromine Trichloride Color bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at 200å c. It is physically stable, but reacts violently with water and most metals and nonmetals. Let us follow a series of steps that will ease our understanding of the methodology to draw lewis structure of brcl3: thallium tribromide can be. Bromine Trichloride Color.
From cartoondealer.com
Br Element Periodic Table On Bromine Color. Vector Illustration Bromine Trichloride Color thallium tribromide can be prepared in ch 3 cn by treating a solution of the monobromide with bromine gas,. bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at. for brcl3, the lewis structure reveals that a total of 28 valence electrons(7 from bromine + 7(3) from 3chlorine atoms) are. Bromine Trichloride Color.
From www.vecteezy.com
Bromine symbol with long shadow design. Chemical element of the Bromine Trichloride Color bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at 200å c. for brcl3, the lewis structure reveals that a total of 28 valence electrons(7 from bromine + 7(3) from 3chlorine atoms) are required to form a single brcl3 molecule. thallium tribromide can be prepared in ch 3 cn by. Bromine Trichloride Color.
From www.chemistore.co.za
Bromine water, 500ml Bromine Trichloride Color bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at. bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at 200å c. for brcl3, the lewis structure reveals that a total of 28 valence electrons(7 from bromine + 7(3) from 3chlorine atoms) are. Bromine Trichloride Color.
From www.slideserve.com
PPT Bromine PowerPoint Presentation, free download ID2813065 Bromine Trichloride Color for brcl3, the lewis structure reveals that a total of 28 valence electrons(7 from bromine + 7(3) from 3chlorine atoms) are required to form a single brcl3 molecule. bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at. thallium tribromide can be prepared in ch 3 cn by treating a. Bromine Trichloride Color.
From www.anyrgb.com
Bromine pentafluoride, phosphorus Tribromide, Nitrogen trichloride Bromine Trichloride Color Let us follow a series of steps that will ease our understanding of the methodology to draw lewis structure of brcl3: bromine pentafluoride (brf 5) is a colorless fuming liquid, made by reacting bromine trifluoride with fluorine at 200å c. for brcl3, the lewis structure reveals that a total of 28 valence electrons(7 from bromine + 7(3) from. Bromine Trichloride Color.