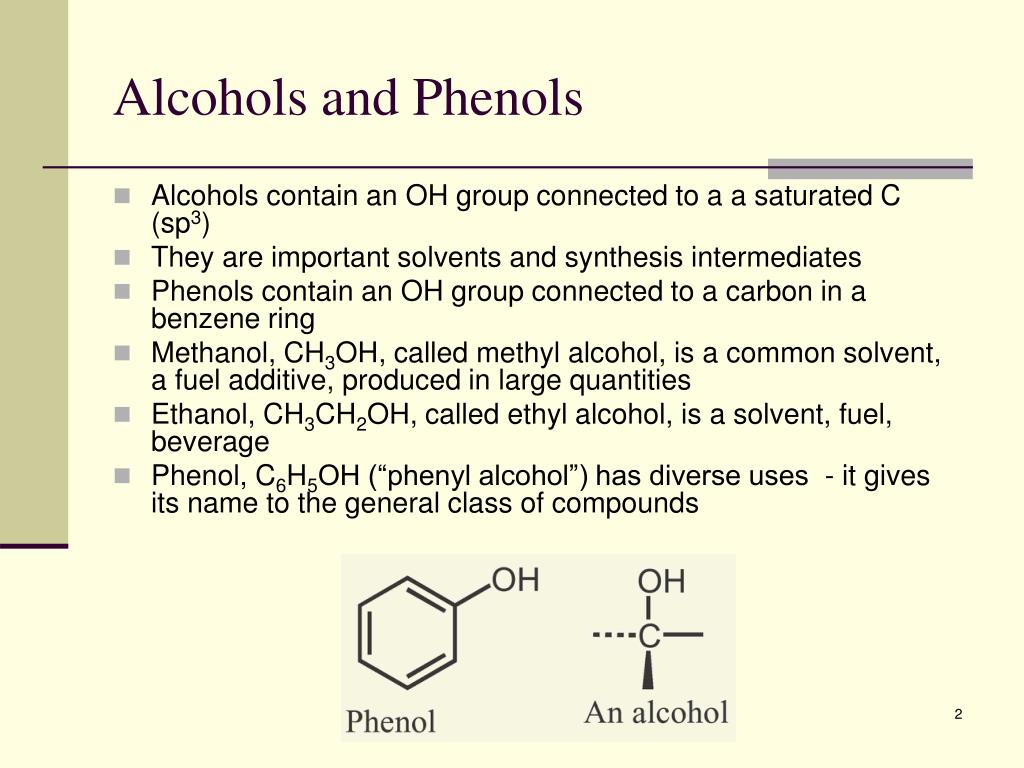
How Do Phenols Differ From Alcohols . It is an exception to the rule that the hydroxyl group must be attached to a. phenol is a type of alcohol where the hydroxyl group is bound to an aromatic ring. both alcohols and phenols are capable of acting as weakly acidic species; alcohols and phenols are organic compounds with at least one hydroxyl group attached to a saturated or. When deprotonated, their conjugate bases. phenols compounds in which an oh group is attached directly to an aromatic ring are designated aroh and. The alcohols are a class of organic compounds that hold. Delocalization of the negative charge over the. phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate.
from www.slideserve.com
alcohols and phenols are organic compounds with at least one hydroxyl group attached to a saturated or. The alcohols are a class of organic compounds that hold. phenols compounds in which an oh group is attached directly to an aromatic ring are designated aroh and. Delocalization of the negative charge over the. phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate. both alcohols and phenols are capable of acting as weakly acidic species; phenol is a type of alcohol where the hydroxyl group is bound to an aromatic ring. When deprotonated, their conjugate bases. It is an exception to the rule that the hydroxyl group must be attached to a.
PPT Alcohols and Phenols PowerPoint Presentation, free download ID
How Do Phenols Differ From Alcohols phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate. both alcohols and phenols are capable of acting as weakly acidic species; phenol is a type of alcohol where the hydroxyl group is bound to an aromatic ring. It is an exception to the rule that the hydroxyl group must be attached to a. phenols compounds in which an oh group is attached directly to an aromatic ring are designated aroh and. alcohols and phenols are organic compounds with at least one hydroxyl group attached to a saturated or. The alcohols are a class of organic compounds that hold. phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate. Delocalization of the negative charge over the. When deprotonated, their conjugate bases.
From studylib.net
Alcohols, Phenols and Ethers How Do Phenols Differ From Alcohols both alcohols and phenols are capable of acting as weakly acidic species; phenol is a type of alcohol where the hydroxyl group is bound to an aromatic ring. The alcohols are a class of organic compounds that hold. alcohols and phenols are organic compounds with at least one hydroxyl group attached to a saturated or. Delocalization of. How Do Phenols Differ From Alcohols.
From www.chegg.com
Solved How do phenols differ from "normal" alcohols? They How Do Phenols Differ From Alcohols When deprotonated, their conjugate bases. The alcohols are a class of organic compounds that hold. It is an exception to the rule that the hydroxyl group must be attached to a. phenols compounds in which an oh group is attached directly to an aromatic ring are designated aroh and. Delocalization of the negative charge over the. phenol is. How Do Phenols Differ From Alcohols.
From www.studypool.com
SOLUTION Alcohols phenols ethers part iii chemistry 2021 Studypool How Do Phenols Differ From Alcohols Delocalization of the negative charge over the. phenol is a type of alcohol where the hydroxyl group is bound to an aromatic ring. alcohols and phenols are organic compounds with at least one hydroxyl group attached to a saturated or. When deprotonated, their conjugate bases. phenols are more acidic than alcohols due to resonance delocalization of the. How Do Phenols Differ From Alcohols.
From slideplayer.com
Alcohols, Ethers and Thiols ppt download How Do Phenols Differ From Alcohols Delocalization of the negative charge over the. both alcohols and phenols are capable of acting as weakly acidic species; When deprotonated, their conjugate bases. It is an exception to the rule that the hydroxyl group must be attached to a. phenol is a type of alcohol where the hydroxyl group is bound to an aromatic ring. phenols. How Do Phenols Differ From Alcohols.
From www.studypool.com
SOLUTION Alcohols phenols ethers part iii chemistry 2021 Studypool How Do Phenols Differ From Alcohols phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate. When deprotonated, their conjugate bases. both alcohols and phenols are capable of acting as weakly acidic species; It is an exception to the rule that the hydroxyl group must be attached to a. phenol is a type of alcohol. How Do Phenols Differ From Alcohols.
From byjus.com
Phenols are more acidic than alcohol. Explain why? How Do Phenols Differ From Alcohols phenol is a type of alcohol where the hydroxyl group is bound to an aromatic ring. phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate. When deprotonated, their conjugate bases. The alcohols are a class of organic compounds that hold. alcohols and phenols are organic compounds with at. How Do Phenols Differ From Alcohols.
From www.slideserve.com
PPT Alcohols and Phenols PowerPoint Presentation, free download ID How Do Phenols Differ From Alcohols The alcohols are a class of organic compounds that hold. phenols compounds in which an oh group is attached directly to an aromatic ring are designated aroh and. Delocalization of the negative charge over the. both alcohols and phenols are capable of acting as weakly acidic species; phenols are more acidic than alcohols due to resonance delocalization. How Do Phenols Differ From Alcohols.
From www.reddit.com
Alcohols Phenols and Ethers, Classification, Nomenclature, And How Do Phenols Differ From Alcohols phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate. both alcohols and phenols are capable of acting as weakly acidic species; phenols compounds in which an oh group is attached directly to an aromatic ring are designated aroh and. phenol is a type of alcohol where the. How Do Phenols Differ From Alcohols.
From www.slideserve.com
PPT Classification and Identification of Alcohols and Phenols How Do Phenols Differ From Alcohols alcohols and phenols are organic compounds with at least one hydroxyl group attached to a saturated or. When deprotonated, their conjugate bases. Delocalization of the negative charge over the. phenols compounds in which an oh group is attached directly to an aromatic ring are designated aroh and. phenol is a type of alcohol where the hydroxyl group. How Do Phenols Differ From Alcohols.
From chemisfast.blogspot.com
Phenol definitionPhenol structure and Identification in chemistry PG How Do Phenols Differ From Alcohols The alcohols are a class of organic compounds that hold. phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate. phenol is a type of alcohol where the hydroxyl group is bound to an aromatic ring. phenols compounds in which an oh group is attached directly to an aromatic. How Do Phenols Differ From Alcohols.
From dokumen.tips
(PDF) Alcohols, Phenols, and Etherschemweb.calpoly.edu/cbailey How Do Phenols Differ From Alcohols When deprotonated, their conjugate bases. Delocalization of the negative charge over the. both alcohols and phenols are capable of acting as weakly acidic species; alcohols and phenols are organic compounds with at least one hydroxyl group attached to a saturated or. phenols compounds in which an oh group is attached directly to an aromatic ring are designated. How Do Phenols Differ From Alcohols.
From www.slideserve.com
PPT Organic Chemistry II Alcohols, Phenols, Thiols , Ethers, and How Do Phenols Differ From Alcohols It is an exception to the rule that the hydroxyl group must be attached to a. The alcohols are a class of organic compounds that hold. phenols compounds in which an oh group is attached directly to an aromatic ring are designated aroh and. phenol is a type of alcohol where the hydroxyl group is bound to an. How Do Phenols Differ From Alcohols.
From thecontentauthority.com
Alcohols vs Phenols Differences And Uses For Each One How Do Phenols Differ From Alcohols alcohols and phenols are organic compounds with at least one hydroxyl group attached to a saturated or. Delocalization of the negative charge over the. both alcohols and phenols are capable of acting as weakly acidic species; The alcohols are a class of organic compounds that hold. phenols are more acidic than alcohols due to resonance delocalization of. How Do Phenols Differ From Alcohols.
From fyoxquzkx.blob.core.windows.net
How To Distinguish Between Alcohol And Phenol at Billy Caldwell blog How Do Phenols Differ From Alcohols The alcohols are a class of organic compounds that hold. phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate. phenol is a type of alcohol where the hydroxyl group is bound to an aromatic ring. alcohols and phenols are organic compounds with at least one hydroxyl group attached. How Do Phenols Differ From Alcohols.
From www.studocu.com
6 Alcohols and Phenols lab report Experiment 6 Alcohols and How Do Phenols Differ From Alcohols phenol is a type of alcohol where the hydroxyl group is bound to an aromatic ring. Delocalization of the negative charge over the. The alcohols are a class of organic compounds that hold. both alcohols and phenols are capable of acting as weakly acidic species; When deprotonated, their conjugate bases. alcohols and phenols are organic compounds with. How Do Phenols Differ From Alcohols.
From www.geeksforgeeks.org
Nomenclature of Alcohols, Phenols and Ethers Rules and Examples How Do Phenols Differ From Alcohols The alcohols are a class of organic compounds that hold. both alcohols and phenols are capable of acting as weakly acidic species; Delocalization of the negative charge over the. phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate. alcohols and phenols are organic compounds with at least one. How Do Phenols Differ From Alcohols.
From www.studypool.com
SOLUTION Alcohols phenols ethers part iii chemistry 2021 Studypool How Do Phenols Differ From Alcohols phenol is a type of alcohol where the hydroxyl group is bound to an aromatic ring. It is an exception to the rule that the hydroxyl group must be attached to a. Delocalization of the negative charge over the. phenols compounds in which an oh group is attached directly to an aromatic ring are designated aroh and. . How Do Phenols Differ From Alcohols.
From byjus.com
7 Phenols can be distinguished from ethyl alcohol by all reagents How Do Phenols Differ From Alcohols phenols compounds in which an oh group is attached directly to an aromatic ring are designated aroh and. phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate. Delocalization of the negative charge over the. alcohols and phenols are organic compounds with at least one hydroxyl group attached to. How Do Phenols Differ From Alcohols.
From readchemistry.com
Structure and Classification of Alcohols Read Chemistry How Do Phenols Differ From Alcohols phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate. The alcohols are a class of organic compounds that hold. phenols compounds in which an oh group is attached directly to an aromatic ring are designated aroh and. alcohols and phenols are organic compounds with at least one hydroxyl. How Do Phenols Differ From Alcohols.
From studylib.net
Alcohols and Phenols How Do Phenols Differ From Alcohols phenol is a type of alcohol where the hydroxyl group is bound to an aromatic ring. When deprotonated, their conjugate bases. Delocalization of the negative charge over the. phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate. alcohols and phenols are organic compounds with at least one hydroxyl. How Do Phenols Differ From Alcohols.
From www.cleariitmedical.com
Alcohols, Phenols and Ethers Chemistry Notes for IITJEE/NEET How Do Phenols Differ From Alcohols alcohols and phenols are organic compounds with at least one hydroxyl group attached to a saturated or. phenols compounds in which an oh group is attached directly to an aromatic ring are designated aroh and. It is an exception to the rule that the hydroxyl group must be attached to a. phenol is a type of alcohol. How Do Phenols Differ From Alcohols.
From www.slideshare.net
Alcohols, Phenols, and Ethers How Do Phenols Differ From Alcohols alcohols and phenols are organic compounds with at least one hydroxyl group attached to a saturated or. phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate. The alcohols are a class of organic compounds that hold. Delocalization of the negative charge over the. It is an exception to the. How Do Phenols Differ From Alcohols.
From byjus.com
What is the order of acidity of alcohols and phenols? Explain both in How Do Phenols Differ From Alcohols It is an exception to the rule that the hydroxyl group must be attached to a. The alcohols are a class of organic compounds that hold. phenol is a type of alcohol where the hydroxyl group is bound to an aromatic ring. Delocalization of the negative charge over the. both alcohols and phenols are capable of acting as. How Do Phenols Differ From Alcohols.
From www.slideserve.com
PPT Alcohols, Phenols, Thiols, and Ethers PowerPoint Presentation How Do Phenols Differ From Alcohols both alcohols and phenols are capable of acting as weakly acidic species; phenols compounds in which an oh group is attached directly to an aromatic ring are designated aroh and. phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate. Delocalization of the negative charge over the. The alcohols. How Do Phenols Differ From Alcohols.
From www.slideserve.com
PPT Alcohols and Phenols PowerPoint Presentation, free download ID How Do Phenols Differ From Alcohols phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate. alcohols and phenols are organic compounds with at least one hydroxyl group attached to a saturated or. Delocalization of the negative charge over the. phenol is a type of alcohol where the hydroxyl group is bound to an aromatic. How Do Phenols Differ From Alcohols.
From www.chem.ucalgary.ca
Chapter 24 Phenols How Do Phenols Differ From Alcohols phenols compounds in which an oh group is attached directly to an aromatic ring are designated aroh and. It is an exception to the rule that the hydroxyl group must be attached to a. alcohols and phenols are organic compounds with at least one hydroxyl group attached to a saturated or. The alcohols are a class of organic. How Do Phenols Differ From Alcohols.
From www.samareducation.com
Alcohols, Phenols and Ethers Class 12 Notes Chemistry Chapter 11 How Do Phenols Differ From Alcohols phenols compounds in which an oh group is attached directly to an aromatic ring are designated aroh and. phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate. alcohols and phenols are organic compounds with at least one hydroxyl group attached to a saturated or. It is an exception. How Do Phenols Differ From Alcohols.
From www.numerade.com
SOLVEDWhy phenols are more acidic than alcohols? How Do Phenols Differ From Alcohols The alcohols are a class of organic compounds that hold. It is an exception to the rule that the hydroxyl group must be attached to a. alcohols and phenols are organic compounds with at least one hydroxyl group attached to a saturated or. Delocalization of the negative charge over the. phenols are more acidic than alcohols due to. How Do Phenols Differ From Alcohols.
From www.slideserve.com
PPT ALCOHOLS and PHENOLS PowerPoint Presentation, free download ID How Do Phenols Differ From Alcohols phenol is a type of alcohol where the hydroxyl group is bound to an aromatic ring. It is an exception to the rule that the hydroxyl group must be attached to a. The alcohols are a class of organic compounds that hold. alcohols and phenols are organic compounds with at least one hydroxyl group attached to a saturated. How Do Phenols Differ From Alcohols.
From www.slideserve.com
PPT Chapter 17 Alcohols and Phenols PowerPoint Presentation, free How Do Phenols Differ From Alcohols both alcohols and phenols are capable of acting as weakly acidic species; Delocalization of the negative charge over the. When deprotonated, their conjugate bases. phenols compounds in which an oh group is attached directly to an aromatic ring are designated aroh and. phenols are more acidic than alcohols due to resonance delocalization of the negative charge in. How Do Phenols Differ From Alcohols.
From www.chegg.com
Solved 1. Explain how alcohols, ethers, and phenols differ How Do Phenols Differ From Alcohols phenol is a type of alcohol where the hydroxyl group is bound to an aromatic ring. phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate. alcohols and phenols are organic compounds with at least one hydroxyl group attached to a saturated or. phenols compounds in which an. How Do Phenols Differ From Alcohols.
From slideplayer.com
Chapter 17 Alcohols and Phenols ppt download How Do Phenols Differ From Alcohols Delocalization of the negative charge over the. The alcohols are a class of organic compounds that hold. both alcohols and phenols are capable of acting as weakly acidic species; phenols compounds in which an oh group is attached directly to an aromatic ring are designated aroh and. phenols are more acidic than alcohols due to resonance delocalization. How Do Phenols Differ From Alcohols.
From www.doubtnut.com
What are phenols? How do they differ structurally from aromatic alcoho How Do Phenols Differ From Alcohols phenols compounds in which an oh group is attached directly to an aromatic ring are designated aroh and. phenol is a type of alcohol where the hydroxyl group is bound to an aromatic ring. It is an exception to the rule that the hydroxyl group must be attached to a. When deprotonated, their conjugate bases. phenols are. How Do Phenols Differ From Alcohols.
From www.slideserve.com
PPT Classification and Identification of Alcohols and Phenols How Do Phenols Differ From Alcohols Delocalization of the negative charge over the. The alcohols are a class of organic compounds that hold. both alcohols and phenols are capable of acting as weakly acidic species; When deprotonated, their conjugate bases. alcohols and phenols are organic compounds with at least one hydroxyl group attached to a saturated or. It is an exception to the rule. How Do Phenols Differ From Alcohols.
From flatworldknowledge.lardbucket.org
Phenols How Do Phenols Differ From Alcohols The alcohols are a class of organic compounds that hold. When deprotonated, their conjugate bases. It is an exception to the rule that the hydroxyl group must be attached to a. phenol is a type of alcohol where the hydroxyl group is bound to an aromatic ring. Delocalization of the negative charge over the. both alcohols and phenols. How Do Phenols Differ From Alcohols.