Lancet Case Report Consent Form . Hence, there is a need for developing a common consent form which is acceptable to all journals. The following statements, if relevant, can be scanned and submitted electronically with your manuscript: Gain permission and written consent to write up the case from the patient or parents, if your patient is a child, and. This form is written according to the discussion document on best practice for consent for publishing. A photocopied, scanned, or faxed copy of the. I give my consent for all or any part of this material to appear in the lancet journals and all editions of the lancet journals, and any other. Statements of role, if any, of. Consent form for case reports. Name of the person signing the consent form. The key elements in the consent form are:
from drlogy.com
Gain permission and written consent to write up the case from the patient or parents, if your patient is a child, and. Hence, there is a need for developing a common consent form which is acceptable to all journals. I give my consent for all or any part of this material to appear in the lancet journals and all editions of the lancet journals, and any other. Name of the person signing the consent form. The key elements in the consent form are: Statements of role, if any, of. A photocopied, scanned, or faxed copy of the. This form is written according to the discussion document on best practice for consent for publishing. The following statements, if relevant, can be scanned and submitted electronically with your manuscript: Consent form for case reports.
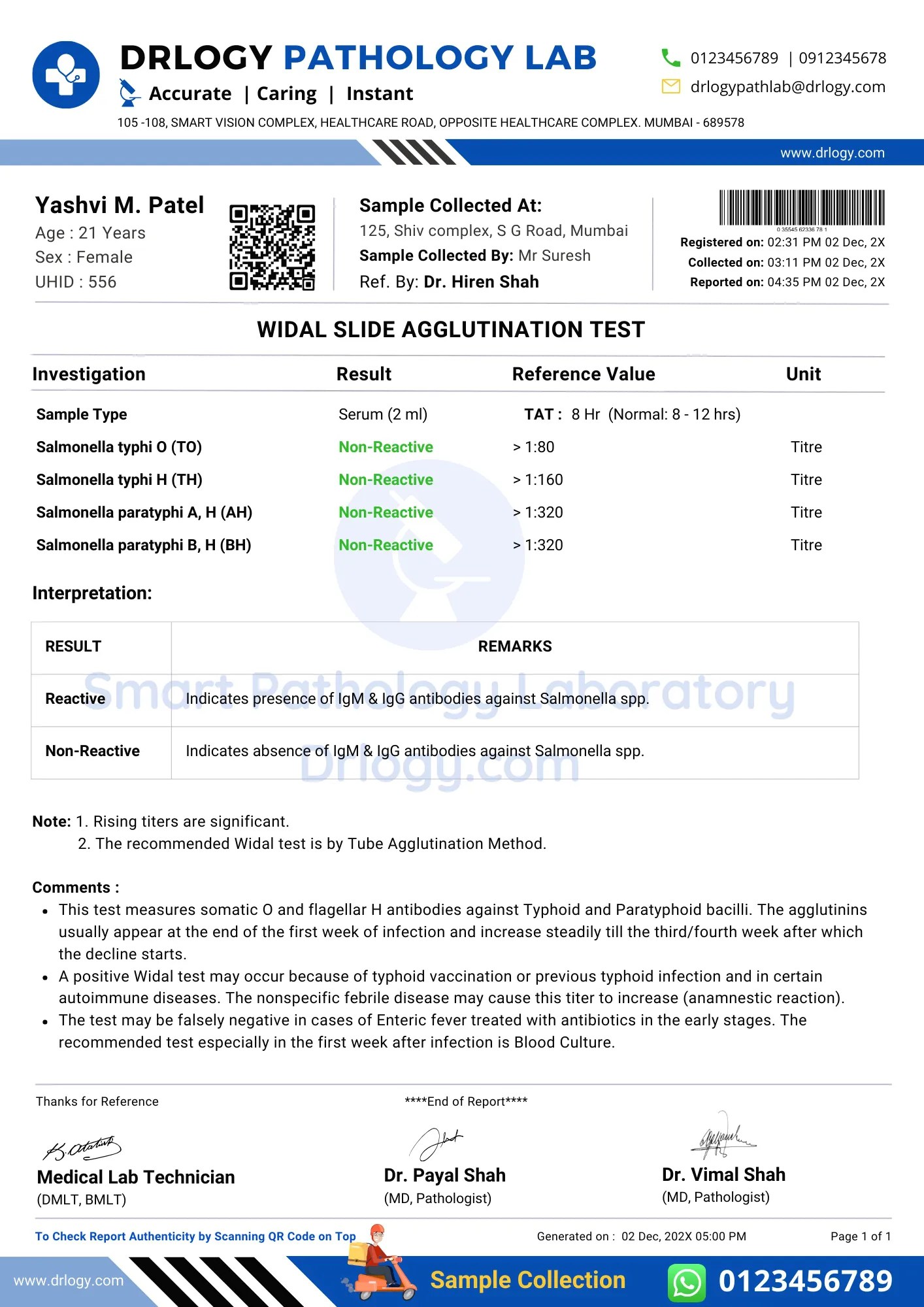
Widal Test Report Format PDF, Sample Template & Example Guide Drlogy
Lancet Case Report Consent Form Gain permission and written consent to write up the case from the patient or parents, if your patient is a child, and. Statements of role, if any, of. This form is written according to the discussion document on best practice for consent for publishing. I give my consent for all or any part of this material to appear in the lancet journals and all editions of the lancet journals, and any other. The key elements in the consent form are: Consent form for case reports. Hence, there is a need for developing a common consent form which is acceptable to all journals. Name of the person signing the consent form. Gain permission and written consent to write up the case from the patient or parents, if your patient is a child, and. A photocopied, scanned, or faxed copy of the. The following statements, if relevant, can be scanned and submitted electronically with your manuscript:
From www.thelancet.com
Anecdotes in medicine—15 years of Lancet Case Reports The Lancet Lancet Case Report Consent Form Name of the person signing the consent form. Statements of role, if any, of. Hence, there is a need for developing a common consent form which is acceptable to all journals. Gain permission and written consent to write up the case from the patient or parents, if your patient is a child, and. Consent form for case reports. This form. Lancet Case Report Consent Form.
From www.thelancet.com
Development and internal validation of a clinical rule to improve Lancet Case Report Consent Form Statements of role, if any, of. Gain permission and written consent to write up the case from the patient or parents, if your patient is a child, and. A photocopied, scanned, or faxed copy of the. Consent form for case reports. The key elements in the consent form are: The following statements, if relevant, can be scanned and submitted electronically. Lancet Case Report Consent Form.
From staging.studyiq.com
Lancet Report on Health Lancet Case Report Consent Form This form is written according to the discussion document on best practice for consent for publishing. The key elements in the consent form are: Statements of role, if any, of. I give my consent for all or any part of this material to appear in the lancet journals and all editions of the lancet journals, and any other. Gain permission. Lancet Case Report Consent Form.
From www.scribd.com
Lancet Information for Authors Clinical Trial Academic Journal Lancet Case Report Consent Form Hence, there is a need for developing a common consent form which is acceptable to all journals. This form is written according to the discussion document on best practice for consent for publishing. Statements of role, if any, of. Name of the person signing the consent form. I give my consent for all or any part of this material to. Lancet Case Report Consent Form.
From www.researchgate.net
(PDF) LANCET CASE REPORT Lancet Case Report Consent Form I give my consent for all or any part of this material to appear in the lancet journals and all editions of the lancet journals, and any other. A photocopied, scanned, or faxed copy of the. This form is written according to the discussion document on best practice for consent for publishing. Name of the person signing the consent form.. Lancet Case Report Consent Form.
From www.thelancet.com
Asthma The Lancet Lancet Case Report Consent Form The following statements, if relevant, can be scanned and submitted electronically with your manuscript: I give my consent for all or any part of this material to appear in the lancet journals and all editions of the lancet journals, and any other. Gain permission and written consent to write up the case from the patient or parents, if your patient. Lancet Case Report Consent Form.
From www.pdffiller.com
Fillable Online MDSLancet Request Form Fax Email Print pdfFiller Lancet Case Report Consent Form I give my consent for all or any part of this material to appear in the lancet journals and all editions of the lancet journals, and any other. This form is written according to the discussion document on best practice for consent for publishing. Statements of role, if any, of. Hence, there is a need for developing a common consent. Lancet Case Report Consent Form.
From www.sampletemplates.com
FREE 12+ Sample Case Report Templates in PDF MS Word Google Docs Lancet Case Report Consent Form Statements of role, if any, of. The following statements, if relevant, can be scanned and submitted electronically with your manuscript: A photocopied, scanned, or faxed copy of the. Name of the person signing the consent form. I give my consent for all or any part of this material to appear in the lancet journals and all editions of the lancet. Lancet Case Report Consent Form.
From fity.club
Article How To Write A Clinical Case Report Lancet Case Report Consent Form This form is written according to the discussion document on best practice for consent for publishing. Consent form for case reports. Statements of role, if any, of. The key elements in the consent form are: A photocopied, scanned, or faxed copy of the. Hence, there is a need for developing a common consent form which is acceptable to all journals.. Lancet Case Report Consent Form.
From www.thelancet.com
Reporting guidelines for clinical trial reports for interventions Lancet Case Report Consent Form Hence, there is a need for developing a common consent form which is acceptable to all journals. The key elements in the consent form are: The following statements, if relevant, can be scanned and submitted electronically with your manuscript: A photocopied, scanned, or faxed copy of the. Statements of role, if any, of. I give my consent for all or. Lancet Case Report Consent Form.
From www.researchgate.net
(PDF) Case reports in the Lancet From neurophobia to global pandemics Lancet Case Report Consent Form The key elements in the consent form are: Name of the person signing the consent form. Consent form for case reports. The following statements, if relevant, can be scanned and submitted electronically with your manuscript: Gain permission and written consent to write up the case from the patient or parents, if your patient is a child, and. Statements of role,. Lancet Case Report Consent Form.
From studylib.net
STATEMENT ON RELEASE OF LANCET COMMISSION REPORT Lancet Case Report Consent Form This form is written according to the discussion document on best practice for consent for publishing. The key elements in the consent form are: Name of the person signing the consent form. A photocopied, scanned, or faxed copy of the. Consent form for case reports. Gain permission and written consent to write up the case from the patient or parents,. Lancet Case Report Consent Form.
From www.thelancet.com
How long does a hip replacement last? A systematic review and meta Lancet Case Report Consent Form Consent form for case reports. The key elements in the consent form are: Statements of role, if any, of. Name of the person signing the consent form. Gain permission and written consent to write up the case from the patient or parents, if your patient is a child, and. A photocopied, scanned, or faxed copy of the. This form is. Lancet Case Report Consent Form.
From templatelab.com
40 Free Notary Acknowledgement & Statement Templates ᐅ TemplateLab Lancet Case Report Consent Form Gain permission and written consent to write up the case from the patient or parents, if your patient is a child, and. The key elements in the consent form are: Name of the person signing the consent form. This form is written according to the discussion document on best practice for consent for publishing. I give my consent for all. Lancet Case Report Consent Form.
From drlogy.com
Widal Test Report Format PDF, Sample Template & Example Guide Drlogy Lancet Case Report Consent Form Hence, there is a need for developing a common consent form which is acceptable to all journals. Consent form for case reports. Gain permission and written consent to write up the case from the patient or parents, if your patient is a child, and. Name of the person signing the consent form. Statements of role, if any, of. The key. Lancet Case Report Consent Form.
From www.thelancet.com
Guidelines for clinical trial protocols for interventions involving Lancet Case Report Consent Form Hence, there is a need for developing a common consent form which is acceptable to all journals. Statements of role, if any, of. Gain permission and written consent to write up the case from the patient or parents, if your patient is a child, and. The key elements in the consent form are: This form is written according to the. Lancet Case Report Consent Form.
From www.pdffiller.com
Fillable Online Patient consent form The Lancet Fax Email Print Lancet Case Report Consent Form I give my consent for all or any part of this material to appear in the lancet journals and all editions of the lancet journals, and any other. Statements of role, if any, of. The key elements in the consent form are: This form is written according to the discussion document on best practice for consent for publishing. A photocopied,. Lancet Case Report Consent Form.
From exopapipl.blob.core.windows.net
Lancet Lab Test Form at Gina Robbs blog Lancet Case Report Consent Form Hence, there is a need for developing a common consent form which is acceptable to all journals. This form is written according to the discussion document on best practice for consent for publishing. Name of the person signing the consent form. The following statements, if relevant, can be scanned and submitted electronically with your manuscript: The key elements in the. Lancet Case Report Consent Form.
From www.globalwomennet.org
The Lancet Commission on lessons for the future from the COVID19 Lancet Case Report Consent Form I give my consent for all or any part of this material to appear in the lancet journals and all editions of the lancet journals, and any other. The key elements in the consent form are: Name of the person signing the consent form. Statements of role, if any, of. The following statements, if relevant, can be scanned and submitted. Lancet Case Report Consent Form.
From www.researchgate.net
(PDF) History of informed medical consent. The lancet. 1995 Oct 7; 346 Lancet Case Report Consent Form The key elements in the consent form are: Consent form for case reports. Statements of role, if any, of. Name of the person signing the consent form. A photocopied, scanned, or faxed copy of the. Gain permission and written consent to write up the case from the patient or parents, if your patient is a child, and. I give my. Lancet Case Report Consent Form.
From www.thelancet.com
Zanidatamab for HER2amplified, unresectable, locally advanced or Lancet Case Report Consent Form Consent form for case reports. I give my consent for all or any part of this material to appear in the lancet journals and all editions of the lancet journals, and any other. A photocopied, scanned, or faxed copy of the. Name of the person signing the consent form. The following statements, if relevant, can be scanned and submitted electronically. Lancet Case Report Consent Form.
From www.thelancet.com
Minimal residual disease responseadapted therapy in newly diagnosed Lancet Case Report Consent Form Hence, there is a need for developing a common consent form which is acceptable to all journals. Statements of role, if any, of. Name of the person signing the consent form. Gain permission and written consent to write up the case from the patient or parents, if your patient is a child, and. The key elements in the consent form. Lancet Case Report Consent Form.
From www.semanticscholar.org
Figure 2 from Anecdotes in medicine—15 years of Lancet Case Reports Lancet Case Report Consent Form The key elements in the consent form are: Gain permission and written consent to write up the case from the patient or parents, if your patient is a child, and. Consent form for case reports. Statements of role, if any, of. The following statements, if relevant, can be scanned and submitted electronically with your manuscript: Name of the person signing. Lancet Case Report Consent Form.
From www.preventionweb.net
The 2022 report of the Lancet Countdown on health and climate change Lancet Case Report Consent Form Gain permission and written consent to write up the case from the patient or parents, if your patient is a child, and. Name of the person signing the consent form. The following statements, if relevant, can be scanned and submitted electronically with your manuscript: This form is written according to the discussion document on best practice for consent for publishing.. Lancet Case Report Consent Form.
From techreport.alayneabrahams.com
How To Write A Case Report Residency Business For College Technical Lancet Case Report Consent Form The key elements in the consent form are: This form is written according to the discussion document on best practice for consent for publishing. Statements of role, if any, of. The following statements, if relevant, can be scanned and submitted electronically with your manuscript: Hence, there is a need for developing a common consent form which is acceptable to all. Lancet Case Report Consent Form.
From www.thelancet.com
How long does a knee replacement last? A systematic review and meta Lancet Case Report Consent Form A photocopied, scanned, or faxed copy of the. Hence, there is a need for developing a common consent form which is acceptable to all journals. The following statements, if relevant, can be scanned and submitted electronically with your manuscript: The key elements in the consent form are: Gain permission and written consent to write up the case from the patient. Lancet Case Report Consent Form.
From www.thelancet.com
Improvement of informed consent and the quality of consent documents Lancet Case Report Consent Form The following statements, if relevant, can be scanned and submitted electronically with your manuscript: Hence, there is a need for developing a common consent form which is acceptable to all journals. I give my consent for all or any part of this material to appear in the lancet journals and all editions of the lancet journals, and any other. This. Lancet Case Report Consent Form.
From www.thelancet.com
Graft dysfunction in compassionate use of engineered pigto Lancet Case Report Consent Form This form is written according to the discussion document on best practice for consent for publishing. Gain permission and written consent to write up the case from the patient or parents, if your patient is a child, and. The key elements in the consent form are: Name of the person signing the consent form. Statements of role, if any, of.. Lancet Case Report Consent Form.
From www.researchgate.net
Baseline patient characteristics reprinted from Lancet Oncology with Lancet Case Report Consent Form This form is written according to the discussion document on best practice for consent for publishing. I give my consent for all or any part of this material to appear in the lancet journals and all editions of the lancet journals, and any other. A photocopied, scanned, or faxed copy of the. Hence, there is a need for developing a. Lancet Case Report Consent Form.
From anthopofagos.blogspot.com
Court Case Brief Template HQ Template Documents Lancet Case Report Consent Form The key elements in the consent form are: Consent form for case reports. A photocopied, scanned, or faxed copy of the. Statements of role, if any, of. Name of the person signing the consent form. Gain permission and written consent to write up the case from the patient or parents, if your patient is a child, and. Hence, there is. Lancet Case Report Consent Form.
From www.thelancet.com
Informed consent procedures for emergency interventional research in Lancet Case Report Consent Form The following statements, if relevant, can be scanned and submitted electronically with your manuscript: Hence, there is a need for developing a common consent form which is acceptable to all journals. Consent form for case reports. Gain permission and written consent to write up the case from the patient or parents, if your patient is a child, and. I give. Lancet Case Report Consent Form.
From www.thelancet.com
How long does a shoulder replacement last? A systematic review and meta Lancet Case Report Consent Form The key elements in the consent form are: Statements of role, if any, of. This form is written according to the discussion document on best practice for consent for publishing. A photocopied, scanned, or faxed copy of the. I give my consent for all or any part of this material to appear in the lancet journals and all editions of. Lancet Case Report Consent Form.
From www.akfc.ca
Ottawa Launch The Lancet Commission Report Aga Khan Foundation Canada Lancet Case Report Consent Form A photocopied, scanned, or faxed copy of the. The key elements in the consent form are: Gain permission and written consent to write up the case from the patient or parents, if your patient is a child, and. Name of the person signing the consent form. I give my consent for all or any part of this material to appear. Lancet Case Report Consent Form.
From www.researchgate.net
Lancet Case Reports by country of origin Download Scientific Diagram Lancet Case Report Consent Form A photocopied, scanned, or faxed copy of the. Statements of role, if any, of. Hence, there is a need for developing a common consent form which is acceptable to all journals. I give my consent for all or any part of this material to appear in the lancet journals and all editions of the lancet journals, and any other. The. Lancet Case Report Consent Form.
From www.youtube.com
The Lancet COVID19 Commission Report Launch YouTube Lancet Case Report Consent Form Statements of role, if any, of. This form is written according to the discussion document on best practice for consent for publishing. Consent form for case reports. The key elements in the consent form are: I give my consent for all or any part of this material to appear in the lancet journals and all editions of the lancet journals,. Lancet Case Report Consent Form.