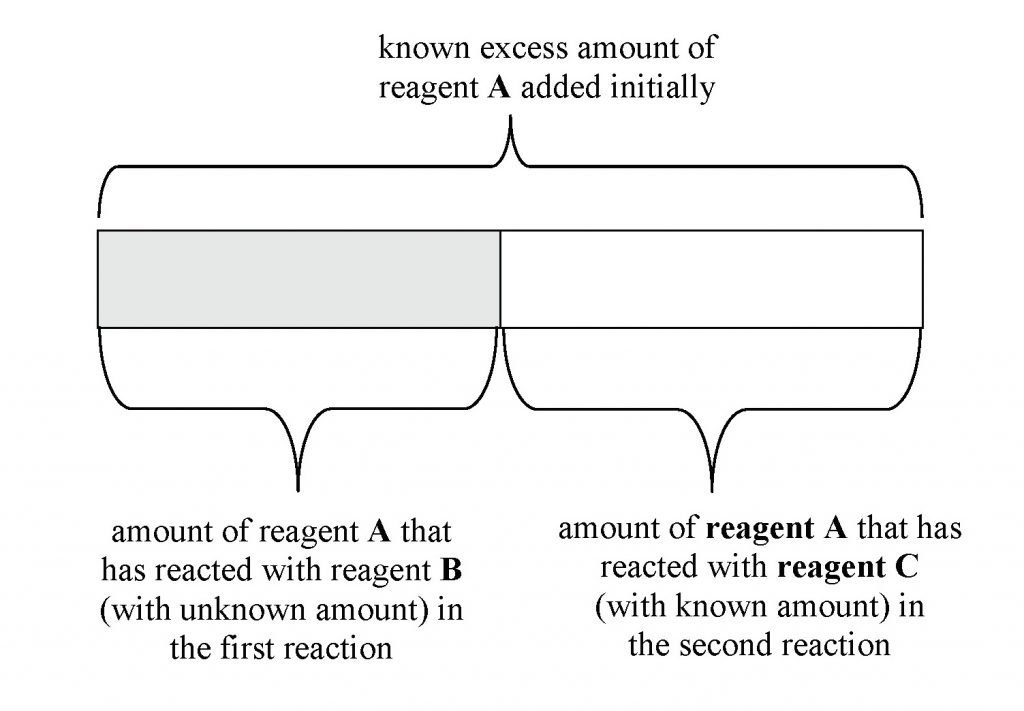
Back Titration Practice Problems . A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. Calculate analyte concentrations given experimental data from a back titration. The mixture was filtered into. In this experiment you will carry out an investigation, calculate the expected mass of product and compare the practical and. Reactant a of unknown concentration is reacted with excess. 4.00g of the limestone was reacted against 200cm 3. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of. You are asked to determine the mass of calcium.
from wealthlesslacas.weebly.com
You are asked to determine the mass of calcium. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of. In this experiment you will carry out an investigation, calculate the expected mass of product and compare the practical and. 4.00g of the limestone was reacted against 200cm 3. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. The mixture was filtered into. Calculate analyte concentrations given experimental data from a back titration. Reactant a of unknown concentration is reacted with excess.
Back Titration Problems And Solutions LINK
Back Titration Practice Problems You are asked to determine the mass of calcium. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of. In this experiment you will carry out an investigation, calculate the expected mass of product and compare the practical and. The mixture was filtered into. Calculate analyte concentrations given experimental data from a back titration. Reactant a of unknown concentration is reacted with excess. You are asked to determine the mass of calcium. 4.00g of the limestone was reacted against 200cm 3.
From www.youtube.com
Acid Base titration problem solving YouTube Back Titration Practice Problems 4.00g of the limestone was reacted against 200cm 3. Reactant a of unknown concentration is reacted with excess. In this experiment you will carry out an investigation, calculate the expected mass of product and compare the practical and. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. The mixture was filtered into. Calculate. Back Titration Practice Problems.
From www.studocu.com
CHEM 110L Experiment 10 It's Back... Titration v2 Experiment 10 It Back Titration Practice Problems The mixture was filtered into. Reactant a of unknown concentration is reacted with excess. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of. In this experiment you will carry out an investigation, calculate the expected. Back Titration Practice Problems.
From www.youtube.com
Chapter 14 part 20, titration practice problem YouTube Back Titration Practice Problems Reactant a of unknown concentration is reacted with excess. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of. 4.00g of the limestone was reacted against 200cm 3. The mixture was filtered into. In this experiment you will carry out an investigation, calculate the expected mass of product and compare the practical. Back Titration Practice Problems.
From www.scribd.com
BackTitration Practice Problems PDF Back Titration Practice Problems 4.00g of the limestone was reacted against 200cm 3. The mixture was filtered into. You are asked to determine the mass of calcium. Calculate analyte concentrations given experimental data from a back titration. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in. Back Titration Practice Problems.
From www.scribd.com
Titrations Practice Problems PDF Back Titration Practice Problems Calculate analyte concentrations given experimental data from a back titration. The mixture was filtered into. In this experiment you will carry out an investigation, calculate the expected mass of product and compare the practical and. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of. 4.00g of the limestone was reacted against. Back Titration Practice Problems.
From wealthlesslacas.weebly.com
Back Titration Problems And Solutions LINK Back Titration Practice Problems A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. 4.00g of the limestone was reacted against 200cm 3. In this experiment you will carry out an investigation, calculate the expected mass of product and compare. Back Titration Practice Problems.
From www.youtube.com
Back Titrations YouTube Back Titration Practice Problems Calculate analyte concentrations given experimental data from a back titration. The mixture was filtered into. In this experiment you will carry out an investigation, calculate the expected mass of product and compare the practical and. Reactant a of unknown concentration is reacted with excess. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. Back Titration Practice Problems.
From www.youtube.com
Back Titrations A level Chemistry YouTube Back Titration Practice Problems A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. Calculate analyte concentrations given experimental data from a back titration. In this experiment you will carry out an investigation, calculate the expected mass of product and compare the practical and. 4.00g of the limestone was reacted against 200cm 3. Reactant a of unknown concentration. Back Titration Practice Problems.
From www.chegg.com
Solved Assignment Back Titration.pdf (1 page) Q Search Back Titration Practice Problems Reactant a of unknown concentration is reacted with excess. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. Calculate analyte concentrations given experimental data from a back titration. In this experiment you will carry out an investigation, calculate the expected mass of product and compare the practical and. A 1.0000 gram sample of. Back Titration Practice Problems.
From www.youtube.com
CHAPTER 5 PART 3A BACK TITRATION 1 YouTube Back Titration Practice Problems The mixture was filtered into. Calculate analyte concentrations given experimental data from a back titration. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. In this experiment you will carry out an investigation, calculate the expected mass of product and compare the practical and. You are asked to determine the mass of calcium.. Back Titration Practice Problems.
From www.youtube.com
Acidbase titration practice problems Stoichiometry YouTube Back Titration Practice Problems 4.00g of the limestone was reacted against 200cm 3. The mixture was filtered into. Reactant a of unknown concentration is reacted with excess. You are asked to determine the mass of calcium. Calculate analyte concentrations given experimental data from a back titration. In this experiment you will carry out an investigation, calculate the expected mass of product and compare the. Back Titration Practice Problems.
From www.studocu.com
Examples of back titration w answers 2008 MCHE223 Studocu Back Titration Practice Problems In this experiment you will carry out an investigation, calculate the expected mass of product and compare the practical and. You are asked to determine the mass of calcium. 4.00g of the limestone was reacted against 200cm 3. Calculate analyte concentrations given experimental data from a back titration. A sample of limestone was analysed to determine the percentage of calcium. Back Titration Practice Problems.
From www.studypool.com
SOLUTION Complex titration with back titration sample problem 25 oct Back Titration Practice Problems Reactant a of unknown concentration is reacted with excess. In this experiment you will carry out an investigation, calculate the expected mass of product and compare the practical and. The mixture was filtered into. 4.00g of the limestone was reacted against 200cm 3. You are asked to determine the mass of calcium. Calculate analyte concentrations given experimental data from a. Back Titration Practice Problems.
From www.slideserve.com
PPT CHAPTER 5 Volumetric Analysis PowerPoint Presentation, free Back Titration Practice Problems Reactant a of unknown concentration is reacted with excess. You are asked to determine the mass of calcium. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. The mixture was filtered into. In this experiment you will carry out an investigation, calculate the expected mass of product and compare the practical and. 4.00g. Back Titration Practice Problems.
From www.youtube.com
Solving AcidBase Titration Problems YouTube Back Titration Practice Problems 4.00g of the limestone was reacted against 200cm 3. Reactant a of unknown concentration is reacted with excess. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of. Calculate analyte concentrations given experimental data from a back titration. In this experiment you will carry out an investigation, calculate the expected mass of. Back Titration Practice Problems.
From www.youtube.com
Back titration practice problem YouTube Back Titration Practice Problems The mixture was filtered into. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. You are asked to determine the mass of calcium. 4.00g of the limestone was reacted against 200cm 3. Calculate analyte concentrations given experimental data from a back titration. In this experiment you will carry out an investigation, calculate the. Back Titration Practice Problems.
From studylib.net
Titration Practice Worksheet Back Titration Practice Problems A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. 4.00g of the limestone was reacted against 200cm 3. In this experiment you will carry out an investigation, calculate the expected mass of product and compare the practical and. You are asked to determine the mass of calcium. Calculate analyte concentrations given experimental data. Back Titration Practice Problems.
From www.youtube.com
Back Titration Calculations // HSC Chemistry YouTube Back Titration Practice Problems 4.00g of the limestone was reacted against 200cm 3. The mixture was filtered into. Calculate analyte concentrations given experimental data from a back titration. In this experiment you will carry out an investigation, calculate the expected mass of product and compare the practical and. Reactant a of unknown concentration is reacted with excess. A 1.0000 gram sample of k2co3 (138.2055. Back Titration Practice Problems.
From www.studypool.com
SOLUTION Example back titration Studypool Back Titration Practice Problems You are asked to determine the mass of calcium. Calculate analyte concentrations given experimental data from a back titration. The mixture was filtered into. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. 4.00g of the limestone was reacted against 200cm 3. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in. Back Titration Practice Problems.
From mavink.com
Back Titration Calculations Back Titration Practice Problems A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. Reactant a of unknown concentration is reacted with excess. The mixture was filtered into. You are asked to determine the mass of calcium. 4.00g of the limestone was reacted against 200cm 3. In this experiment you will carry out an investigation, calculate the expected. Back Titration Practice Problems.
From www.scribd.com
Back Titration Example Problem PDF Back Titration Practice Problems Calculate analyte concentrations given experimental data from a back titration. 4.00g of the limestone was reacted against 200cm 3. You are asked to determine the mass of calcium. Reactant a of unknown concentration is reacted with excess. In this experiment you will carry out an investigation, calculate the expected mass of product and compare the practical and. A 1.0000 gram. Back Titration Practice Problems.
From www.youtube.com
Back Titrations Worked Examples YouTube Back Titration Practice Problems 4.00g of the limestone was reacted against 200cm 3. In this experiment you will carry out an investigation, calculate the expected mass of product and compare the practical and. Calculate analyte concentrations given experimental data from a back titration. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of. You are asked. Back Titration Practice Problems.
From www.youtube.com
Back Titration Explained YouTube Back Titration Practice Problems A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of. You are asked to determine the mass of calcium. In this experiment you will carry out an investigation, calculate the expected mass of product and compare the practical and. A sample of limestone was analysed to determine the percentage of calcium carbonate. Back Titration Practice Problems.
From www.youtube.com
Back Titrations Example YouTube Back Titration Practice Problems 4.00g of the limestone was reacted against 200cm 3. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of. You are asked to determine the mass of calcium. Calculate analyte concentrations given experimental data from a. Back Titration Practice Problems.
From www.youtube.com
Back Titration Example YouTube Back Titration Practice Problems Reactant a of unknown concentration is reacted with excess. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. Calculate analyte concentrations given experimental data from a back titration. The mixture was filtered into. You are asked to determine the mass of calcium. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in. Back Titration Practice Problems.
From www.studocu.com
17 Back Titrations edddddcd Back Titrations Question 1 A 10 g piece Back Titration Practice Problems You are asked to determine the mass of calcium. The mixture was filtered into. In this experiment you will carry out an investigation, calculate the expected mass of product and compare the practical and. Calculate analyte concentrations given experimental data from a back titration. Reactant a of unknown concentration is reacted with excess. A sample of limestone was analysed to. Back Titration Practice Problems.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Back Titration Practice Problems Calculate analyte concentrations given experimental data from a back titration. 4.00g of the limestone was reacted against 200cm 3. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of. You are asked to determine the mass of calcium. The mixture was filtered into. Reactant a of unknown concentration is reacted with excess.. Back Titration Practice Problems.
From www.youtube.com
Back Titration Experiment Explained YouTube Back Titration Practice Problems A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. Reactant a of unknown concentration is reacted with excess. The mixture was filtered into. You are asked to determine the mass of calcium. In this experiment. Back Titration Practice Problems.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Back Titration Practice Problems The mixture was filtered into. 4.00g of the limestone was reacted against 200cm 3. Calculate analyte concentrations given experimental data from a back titration. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. Reactant a. Back Titration Practice Problems.
From kit.co
Back Titration Problems With Answers Back Titration Practice Problems Calculate analyte concentrations given experimental data from a back titration. In this experiment you will carry out an investigation, calculate the expected mass of product and compare the practical and. You are asked to determine the mass of calcium. Reactant a of unknown concentration is reacted with excess. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough. Back Titration Practice Problems.
From mungfali.com
Titration Example Problem Back Titration Practice Problems A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. 4.00g of the limestone was reacted against 200cm 3. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of. Calculate analyte concentrations given experimental data from a back titration. In this experiment you will carry out. Back Titration Practice Problems.
From printablelibfinance.z13.web.core.windows.net
Titration Practice Problems With Answers Pdf Back Titration Practice Problems You are asked to determine the mass of calcium. Reactant a of unknown concentration is reacted with excess. 4.00g of the limestone was reacted against 200cm 3. A sample of limestone was analysed to determine the percentage of calcium carbonate in the rock. Calculate analyte concentrations given experimental data from a back titration. The mixture was filtered into. In this. Back Titration Practice Problems.
From lessonmagicchampart.z14.web.core.windows.net
Titration Math Practice Problems Back Titration Practice Problems Calculate analyte concentrations given experimental data from a back titration. You are asked to determine the mass of calcium. Reactant a of unknown concentration is reacted with excess. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of. In this experiment you will carry out an investigation, calculate the expected mass of. Back Titration Practice Problems.
From www.youtube.com
5 Solving Titration problems with acids and bases YouTube Back Titration Practice Problems A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of. Calculate analyte concentrations given experimental data from a back titration. Reactant a of unknown concentration is reacted with excess. 4.00g of the limestone was reacted against 200cm 3. A sample of limestone was analysed to determine the percentage of calcium carbonate in. Back Titration Practice Problems.
From www.youtube.com
How to solve question Of Back titration? YouTube Back Titration Practice Problems You are asked to determine the mass of calcium. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of. In this experiment you will carry out an investigation, calculate the expected mass of product and compare the practical and. A sample of limestone was analysed to determine the percentage of calcium carbonate. Back Titration Practice Problems.