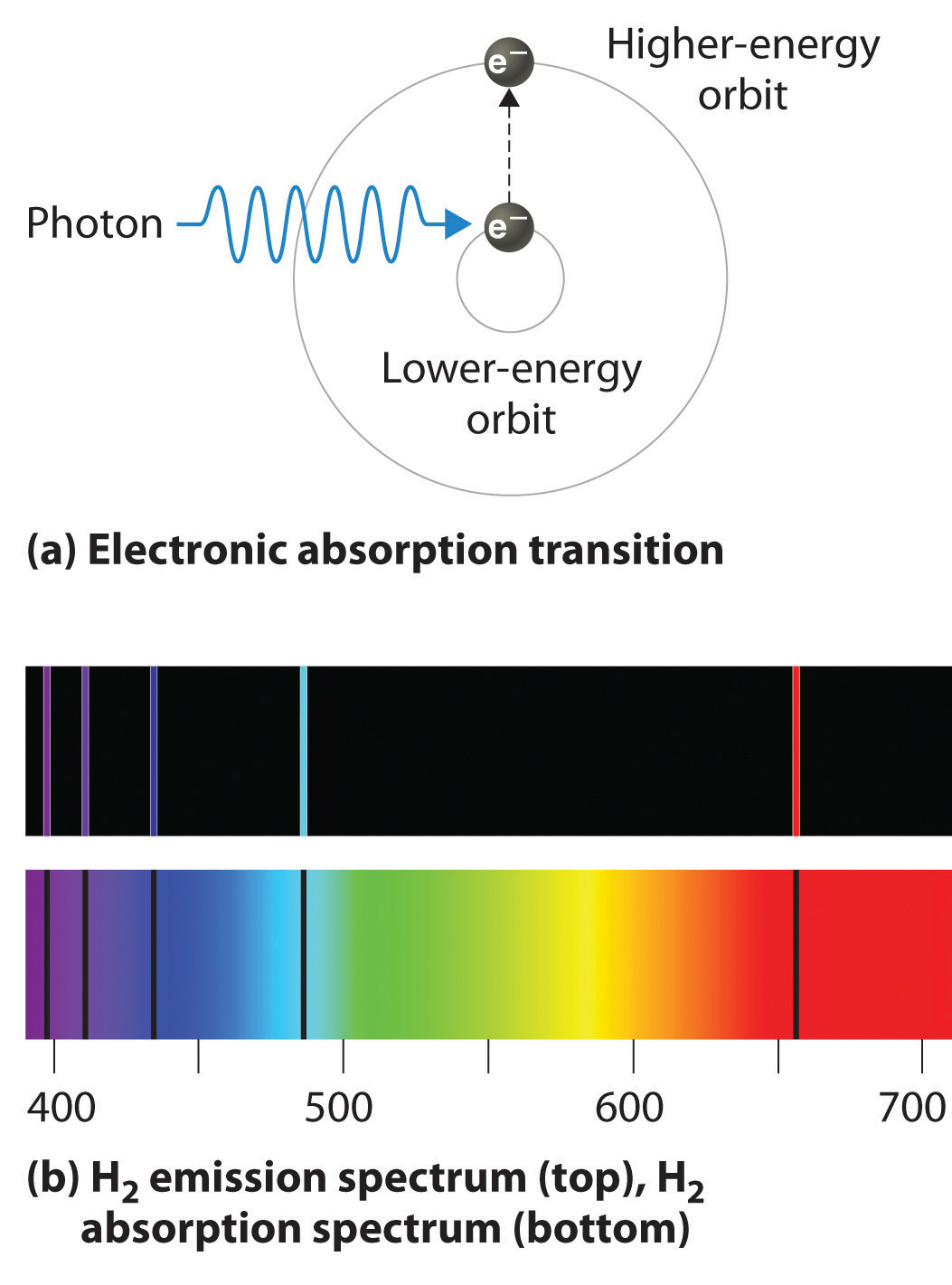
Emission Spectra Definition . The result is an emission spectrum that shows the intensity of emission as a function of wavelength. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. Emission spectra are patterns of light emitted by atoms or molecules when they transition from a higher energy state to a lower energy state. An emission spectrum is unique to each element. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation. Learn what is emission spectrum, how it is formed by atoms and molecules, and how it differs from absorption spectrum. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. The figure below shows the atomic emission spectrum of hydrogen. The emission spectrum of burning fuel or other molecules may also be used to example its composition.
from chem.libretexts.org
Emission spectra are patterns of light emitted by atoms or molecules when they transition from a higher energy state to a lower energy state. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation. An emission spectrum is unique to each element. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Learn what is emission spectrum, how it is formed by atoms and molecules, and how it differs from absorption spectrum. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The emission spectrum of burning fuel or other molecules may also be used to example its composition. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current.
7.3 Atomic Emission Spectra and the Bohr Model Chemistry LibreTexts
Emission Spectra Definition When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The figure below shows the atomic emission spectrum of hydrogen. An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation. Learn what is emission spectrum, how it is formed by atoms and molecules, and how it differs from absorption spectrum. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Emission spectra are patterns of light emitted by atoms or molecules when they transition from a higher energy state to a lower energy state. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. An emission spectrum is unique to each element. The emission spectrum of burning fuel or other molecules may also be used to example its composition.
From study.com
Continuous Spectrum Definition, Types & Examples Lesson Emission Spectra Definition The figure below shows the atomic emission spectrum of hydrogen. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation. An emission spectrum is unique to each element. An atomic emission spectrum. Emission Spectra Definition.
From imagine.gsfc.nasa.gov
Spectra Introduction Emission Spectra Definition Emission spectra are patterns of light emitted by atoms or molecules when they transition from a higher energy state to a lower energy state. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. An atomic emission spectrum is the pattern of lines formed. Emission Spectra Definition.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum Emission Spectra Definition An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. An emission spectrum is unique to each element. The figure below shows the atomic emission spectrum of hydrogen. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of. Emission Spectra Definition.
From byjus.com
Atomic Spectra (Emission Spectrum & Absorption Spectra) Detailed Emission Spectra Definition In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. An emission spectrum is unique to each element. The emission spectrum of burning fuel or other molecules may also be used to example its composition. The result is an emission spectrum that shows the intensity of. Emission Spectra Definition.
From educationgrafts.z21.web.core.windows.net
Line Emission Spectrum Emission Spectra Definition The emission spectrum of burning fuel or other molecules may also be used to example its composition. The figure below shows the atomic emission spectrum of hydrogen. Learn what is emission spectrum, how it is formed by atoms and molecules, and how it differs from absorption spectrum. An atomic emission spectrum is the pattern of lines formed when light passes. Emission Spectra Definition.
From www.astronoo.com
Spectroscopy — Astronoo Emission Spectra Definition An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. An emission spectrum is the characteristic pattern. Emission Spectra Definition.
From ucscphysicsdemo.sites.ucsc.edu
Linear Spectra UCSC Physics Demonstration Room Emission Spectra Definition The figure below shows the atomic emission spectrum of hydrogen. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current.. Emission Spectra Definition.
From whitsonmysecutage.blogspot.com
Continuous Spectrum Vs Emission Spectrum Vs Absorption Spectrum Emission Spectra Definition Learn what is emission spectrum, how it is formed by atoms and molecules, and how it differs from absorption spectrum. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. Emission spectra are patterns of light emitted by atoms or molecules when they transition from a higher energy state. Emission Spectra Definition.
From gamesmartz.com
Atomic Emission Spectrum Definition & Image GameSmartz Emission Spectra Definition An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Learn what is emission spectrum, how it. Emission Spectra Definition.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts Emission Spectra Definition An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation. Learn what is emission spectrum, how it is formed by atoms and molecules, and how it differs from absorption spectrum. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it. Emission Spectra Definition.
From www.sciencelearn.org.nz
The spectrum — Science Learning Hub Emission Spectra Definition When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The figure below shows the atomic emission spectrum of hydrogen. Learn what. Emission Spectra Definition.
From rightmetal.weebly.com
Atomic emission spectrum chemistry definition rightmetal Emission Spectra Definition The emission spectrum of burning fuel or other molecules may also be used to example its composition. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the. Emission Spectra Definition.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Emission Spectra Definition When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light. Emission Spectra Definition.
From www.slideserve.com
PPT Atomic Emission Spectrum PowerPoint Presentation, free download Emission Spectra Definition The emission spectrum of burning fuel or other molecules may also be used to example its composition. Learn what is emission spectrum, how it is formed by atoms and molecules, and how it differs from absorption spectrum. Emission spectra are patterns of light emitted by atoms or molecules when they transition from a higher energy state to a lower energy. Emission Spectra Definition.
From www.thoughtco.com
Balmer Series Definition in Science Emission Spectra Definition When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. The emission spectrum of burning fuel or other molecules may also be used to example its composition. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or. Emission Spectra Definition.
From www.slideserve.com
PPT EMISSION AND ABSORPTION SPECTRA PowerPoint Presentation, free Emission Spectra Definition The figure below shows the atomic emission spectrum of hydrogen. The emission spectrum of burning fuel or other molecules may also be used to example its composition. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. The emission spectrum (or line spectrum) of a chemical element is the. Emission Spectra Definition.
From www.slideserve.com
PPT Lab 5 Emission Spectra PowerPoint Presentation, free download Emission Spectra Definition Emission spectra are patterns of light emitted by atoms or molecules when they transition from a higher energy state to a lower energy state. An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation. An emission spectrum is unique to each element. An atomic emission spectrum is the pattern of lines formed when light passes through. Emission Spectra Definition.
From adawyaf.blogspot.com
Chemistry Grade 9, Atomic Emission Spectra , Introduction Emission Spectra Definition Learn what is emission spectrum, how it is formed by atoms and molecules, and how it differs from absorption spectrum. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. The figure below shows the atomic emission spectrum of hydrogen. Emission spectra are patterns of light emitted by atoms or molecules when they. Emission Spectra Definition.
From www.slideserve.com
PPT Lecture 12 Introduction to Atomic Spectroscopy PowerPoint Emission Spectra Definition An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. When hydrogen gas is placed into a tube and. Emission Spectra Definition.
From www.slideshare.net
Emission spectroscopy Emission Spectra Definition An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation. Emission spectra are patterns of light emitted by atoms or molecules when they transition from a higher energy state to a lower energy state. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different. Emission Spectra Definition.
From chem.libretexts.org
5.5 Atomic Emission Spectra Chemistry LibreTexts Emission Spectra Definition An emission spectrum is unique to each element. Learn what is emission spectrum, how it is formed by atoms and molecules, and how it differs from absorption spectrum. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. An atomic emission spectrum is the pattern of lines formed when. Emission Spectra Definition.
From webbtelescope.org
Types of Spectra Continuous, Emission, and Absorption b Emission Spectra Definition An emission spectrum is unique to each element. Learn what is emission spectrum, how it is formed by atoms and molecules, and how it differs from absorption spectrum. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of. Emission Spectra Definition.
From stock.adobe.com
Continuous spectrum, an emission spectrum that consists of continuum of Emission Spectra Definition The result is an emission spectrum that shows the intensity of emission as a function of wavelength. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. An emission spectrum is unique to each element. The figure below shows the atomic emission spectrum of hydrogen. In chemistry, an emission. Emission Spectra Definition.
From chem.libretexts.org
7.3 Atomic Emission Spectra and the Bohr Model Chemistry LibreTexts Emission Spectra Definition An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The figure below shows the atomic emission spectrum of hydrogen. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. An emission spectrum is the characteristic. Emission Spectra Definition.
From saylordotorg.github.io
Atomic Spectra and Models of the Atom Emission Spectra Definition Emission spectra are patterns of light emitted by atoms or molecules when they transition from a higher energy state to a lower energy state. An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. In chemistry, an emission spectrum. Emission Spectra Definition.
From www.sciencefacts.net
Atomic Emission and Absorption Spectra Definition and Formula Emission Spectra Definition Emission spectra are patterns of light emitted by atoms or molecules when they transition from a higher energy state to a lower energy state. An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected. Emission Spectra Definition.
From www.comsol.com
Calculating the Emission Spectra from Common Light Sources COMSOL Blog Emission Spectra Definition An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. An emission spectrum is unique to each element.. Emission Spectra Definition.
From www.priyamstudycentre.com
Spectroscopy Definition, Types, Applications Emission Spectra Definition When hydrogen gas is placed into a tube and electric current passed through it, the color of emitted light is pink. The emission spectrum of burning fuel or other molecules may also be used to example its composition. An emission spectrum is unique to each element. The result is an emission spectrum that shows the intensity of emission as a. Emission Spectra Definition.
From www.youtube.com
Spectrum Basic Introduction YouTube Emission Spectra Definition Emission spectra are patterns of light emitted by atoms or molecules when they transition from a higher energy state to a lower energy state. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Learn what is emission spectrum, how it is formed by atoms and molecules, and how it differs from absorption. Emission Spectra Definition.
From quizzfullmarks77.z19.web.core.windows.net
Atomic Emission Spectrum Definition Chemistry Emission Spectra Definition An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. Learn what is emission spectrum, how it is formed by atoms and molecules, and how it differs from absorption. Emission Spectra Definition.
From www.slideserve.com
PPT PreIB/PreAP CHEMISTRY PowerPoint Presentation, free download Emission Spectra Definition The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation. When hydrogen gas. Emission Spectra Definition.
From www.youtube.com
Emission and absorption spectrum (class xi chemistry) YouTube Emission Spectra Definition The emission spectrum of burning fuel or other molecules may also be used to example its composition. An emission spectrum is unique to each element. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When hydrogen gas is placed into a tube and. Emission Spectra Definition.
From montessorimuddle.org
Emission Spectra How Atoms Emit and Absorb Light Montessori Muddle Emission Spectra Definition The emission spectrum of burning fuel or other molecules may also be used to example its composition. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. An emission spectrum is unique to each element. An emission spectrum is the characteristic pattern of wavelengths or frequencies of electromagnetic radiation. In chemistry, an emission. Emission Spectra Definition.
From sekaangry.weebly.com
Atomic emission spectrum chemistry definition sekaangry Emission Spectra Definition The emission spectrum of burning fuel or other molecules may also be used to example its composition. Learn what is emission spectrum, how it is formed by atoms and molecules, and how it differs from absorption spectrum. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies. Emission Spectra Definition.
From www.pinterest.com
Image of absorption, emission, and continuous spectra. Absorption Emission Spectra Definition An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. An emission spectrum is unique to each element. Learn what is emission spectrum, how it is formed by atoms and molecules, and how it differs from absorption spectrum. In chemistry, an emission spectrum. Emission Spectra Definition.