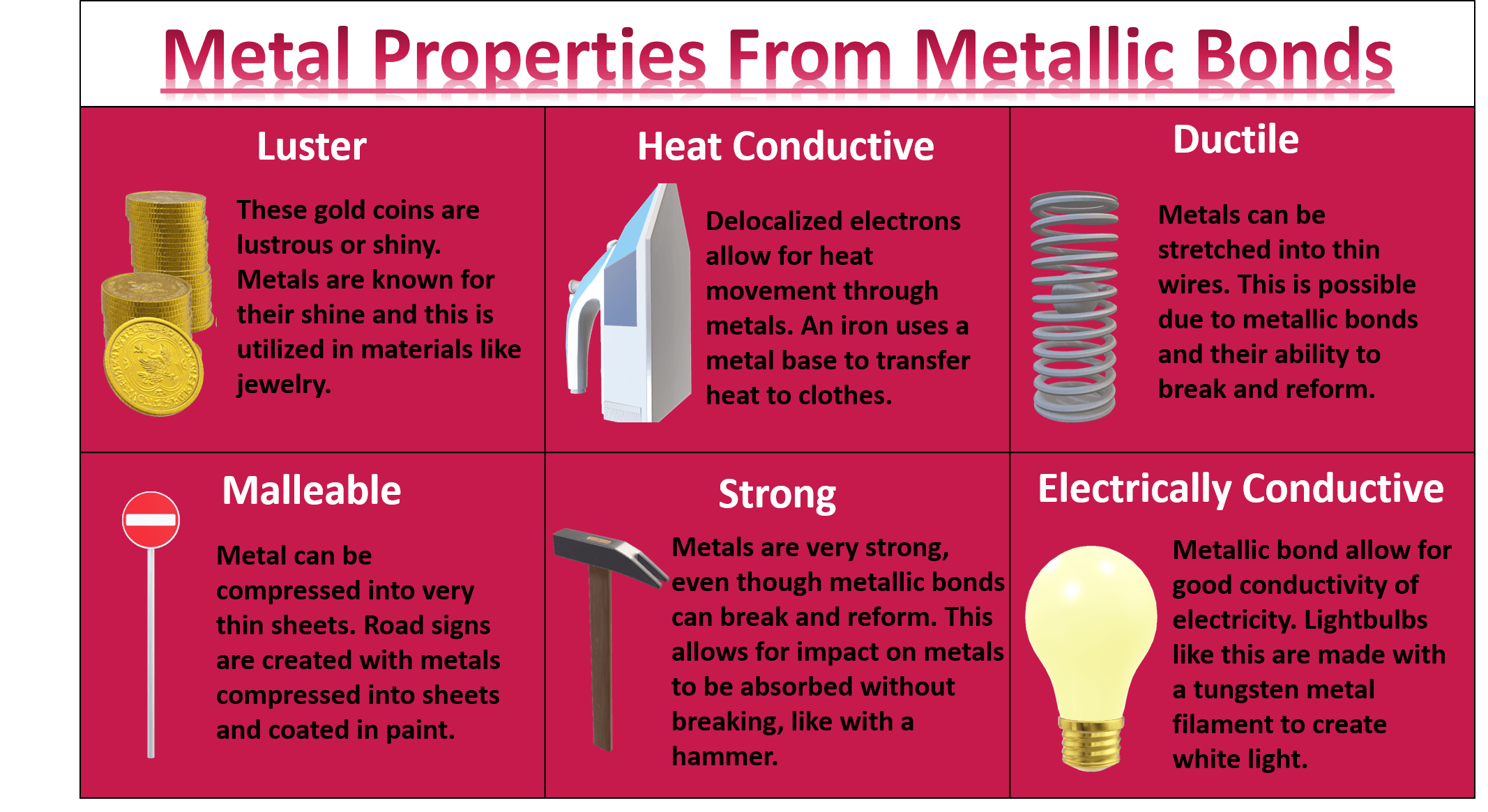
Why Are Metals Malleable In Terms Of Structure And Bonding . The attractive forces between the metal ions. The structure of metals explains their high melting and boiling points and their conductivity. The bonding that occurs in a metal is responsible for its distinctive properties: When a force is applied, the metal layers can slide. Metallic bonding & giant metallic lattice structures. Metallic bonds involve all of the metal atoms. The metallic bond is not fully broken until the metal boils. Metals are malleable and ductile because of metallic bonding. The properties of a metal can be modified by mixing it with another substance to form an alloy. Luster, malleability, ductility, and excellent conductivity. Most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. Metallic bonds are very strong so the giant metallic structure is strongly held together: Metallic bonding is different from ionic and covalent bonding. Metallic bonding is it's own type of bond. In a molten metal, the metallic bond is still present, although the ordered structure has been broken down.
from www.expii.com
The structure of metals explains their high melting and boiling points and their conductivity. Luster, malleability, ductility, and excellent conductivity. Metallic bonds are very strong so the giant metallic structure is strongly held together: The bonding that occurs in a metal is responsible for its distinctive properties: The attractive forces between the metal ions. Most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. Metallic bonds involve all of the metal atoms. The metallic bond is not fully broken until the metal boils. Metallic bonding is different from ionic and covalent bonding. Metallic bonding is it's own type of bond.
Metallic Bond — Formation & Compounds Expii
Why Are Metals Malleable In Terms Of Structure And Bonding Metallic bonding is different from ionic and covalent bonding. The properties of a metal can be modified by mixing it with another substance to form an alloy. In a molten metal, the metallic bond is still present, although the ordered structure has been broken down. Metallic bonds involve all of the metal atoms. Metallic bonding & giant metallic lattice structures. The metallic bond is not fully broken until the metal boils. Metallic bonds are very strong so the giant metallic structure is strongly held together: Metallic bonding is it's own type of bond. The attractive forces between the metal ions. The structure of metals explains their high melting and boiling points and their conductivity. Metallic bonding is different from ionic and covalent bonding. Metals are malleable and ductile because of metallic bonding. The bonding that occurs in a metal is responsible for its distinctive properties: Luster, malleability, ductility, and excellent conductivity. Most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. When a force is applied, the metal layers can slide.
From www.slideserve.com
PPT Structure and bonding in metals PowerPoint Presentation, free Why Are Metals Malleable In Terms Of Structure And Bonding Metallic bonding & giant metallic lattice structures. The properties of a metal can be modified by mixing it with another substance to form an alloy. Luster, malleability, ductility, and excellent conductivity. Metallic bonds are very strong so the giant metallic structure is strongly held together: The bonding that occurs in a metal is responsible for its distinctive properties: The metallic. Why Are Metals Malleable In Terms Of Structure And Bonding.
From slideplayer.com
KS4 Chemistry Metallic Bonding. ppt download Why Are Metals Malleable In Terms Of Structure And Bonding Metallic bonds involve all of the metal atoms. When a force is applied, the metal layers can slide. Luster, malleability, ductility, and excellent conductivity. Metallic bonding is it's own type of bond. In a molten metal, the metallic bond is still present, although the ordered structure has been broken down. Most metals are malleable because the atoms can roll over. Why Are Metals Malleable In Terms Of Structure And Bonding.
From www.slideserve.com
PPT Metallic bonding and properties PowerPoint Presentation, free Why Are Metals Malleable In Terms Of Structure And Bonding The properties of a metal can be modified by mixing it with another substance to form an alloy. The attractive forces between the metal ions. Metallic bonding & giant metallic lattice structures. Metallic bonding is it's own type of bond. Luster, malleability, ductility, and excellent conductivity. Metals are malleable and ductile because of metallic bonding. Metallic bonds are very strong. Why Are Metals Malleable In Terms Of Structure And Bonding.
From slidetodoc.com
Metallic Bonding and Structure Describe metallic bonding as Why Are Metals Malleable In Terms Of Structure And Bonding The attractive forces between the metal ions. Metallic bonding is it's own type of bond. Most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. The metallic bond is not fully broken until the metal boils. In a molten metal, the metallic bond is still present, although the ordered structure has. Why Are Metals Malleable In Terms Of Structure And Bonding.
From slideplayer.com
Ions, Charges, and Metallic Bonds ppt download Why Are Metals Malleable In Terms Of Structure And Bonding Metallic bonding is different from ionic and covalent bonding. The attractive forces between the metal ions. Metallic bonds involve all of the metal atoms. Metals are malleable and ductile because of metallic bonding. The properties of a metal can be modified by mixing it with another substance to form an alloy. When a force is applied, the metal layers can. Why Are Metals Malleable In Terms Of Structure And Bonding.
From byjus.com
Why are metals malleable, ductile, sonorus, lustrous and why are group Why Are Metals Malleable In Terms Of Structure And Bonding Luster, malleability, ductility, and excellent conductivity. The bonding that occurs in a metal is responsible for its distinctive properties: Metallic bonds involve all of the metal atoms. Metallic bonds are very strong so the giant metallic structure is strongly held together: When a force is applied, the metal layers can slide. The properties of a metal can be modified by. Why Are Metals Malleable In Terms Of Structure And Bonding.
From www.slideserve.com
PPT Chapter 7 “Metallic Bonding” PowerPoint Presentation, free Why Are Metals Malleable In Terms Of Structure And Bonding Metallic bonding is different from ionic and covalent bonding. Metals are malleable and ductile because of metallic bonding. The properties of a metal can be modified by mixing it with another substance to form an alloy. The attractive forces between the metal ions. The structure of metals explains their high melting and boiling points and their conductivity. Luster, malleability, ductility,. Why Are Metals Malleable In Terms Of Structure And Bonding.
From www.slideserve.com
PPT Metallic Bonding PowerPoint Presentation, free download ID2696346 Why Are Metals Malleable In Terms Of Structure And Bonding The properties of a metal can be modified by mixing it with another substance to form an alloy. The structure of metals explains their high melting and boiling points and their conductivity. When a force is applied, the metal layers can slide. The metallic bond is not fully broken until the metal boils. Metallic bonding & giant metallic lattice structures.. Why Are Metals Malleable In Terms Of Structure And Bonding.
From www.slideserve.com
PPT Metallic Bonding PowerPoint Presentation, free download ID2041377 Why Are Metals Malleable In Terms Of Structure And Bonding Metallic bonding & giant metallic lattice structures. The attractive forces between the metal ions. The bonding that occurs in a metal is responsible for its distinctive properties: Metallic bonding is it's own type of bond. Metallic bonds involve all of the metal atoms. The properties of a metal can be modified by mixing it with another substance to form an. Why Are Metals Malleable In Terms Of Structure And Bonding.
From www.slideserve.com
PPT 4.4 Metallic bonding PowerPoint Presentation ID400526 Why Are Metals Malleable In Terms Of Structure And Bonding The bonding that occurs in a metal is responsible for its distinctive properties: In a molten metal, the metallic bond is still present, although the ordered structure has been broken down. The structure of metals explains their high melting and boiling points and their conductivity. When a force is applied, the metal layers can slide. Metallic bonding is different from. Why Are Metals Malleable In Terms Of Structure And Bonding.
From www.science-revision.co.uk
Metallic bonding Why Are Metals Malleable In Terms Of Structure And Bonding The metallic bond is not fully broken until the metal boils. The attractive forces between the metal ions. In a molten metal, the metallic bond is still present, although the ordered structure has been broken down. Metals are malleable and ductile because of metallic bonding. The structure of metals explains their high melting and boiling points and their conductivity. Luster,. Why Are Metals Malleable In Terms Of Structure And Bonding.
From ar.inspiredpencil.com
Metallic Bonds Malleable Why Are Metals Malleable In Terms Of Structure And Bonding Metallic bonds involve all of the metal atoms. Metallic bonding is it's own type of bond. Metallic bonding is different from ionic and covalent bonding. The metallic bond is not fully broken until the metal boils. When a force is applied, the metal layers can slide. Metals are malleable and ductile because of metallic bonding. The structure of metals explains. Why Are Metals Malleable In Terms Of Structure And Bonding.
From www.slideserve.com
PPT Unit 4 Metallic Bonding PowerPoint Presentation, free download Why Are Metals Malleable In Terms Of Structure And Bonding Metallic bonds are very strong so the giant metallic structure is strongly held together: The structure of metals explains their high melting and boiling points and their conductivity. Metallic bonds involve all of the metal atoms. The attractive forces between the metal ions. Luster, malleability, ductility, and excellent conductivity. Metallic bonding & giant metallic lattice structures. The properties of a. Why Are Metals Malleable In Terms Of Structure And Bonding.
From www.slideshare.net
Metals Why Are Metals Malleable In Terms Of Structure And Bonding Metallic bonds are very strong so the giant metallic structure is strongly held together: Most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. Metallic bonding & giant metallic lattice structures. The attractive forces between the metal ions. In a molten metal, the metallic bond is still present, although the ordered. Why Are Metals Malleable In Terms Of Structure And Bonding.
From www.slideserve.com
PPT Metals PowerPoint Presentation, free download ID6413590 Why Are Metals Malleable In Terms Of Structure And Bonding The attractive forces between the metal ions. When a force is applied, the metal layers can slide. The structure of metals explains their high melting and boiling points and their conductivity. Metallic bonds are very strong so the giant metallic structure is strongly held together: Most metals are malleable because the atoms can roll over each other and retain the. Why Are Metals Malleable In Terms Of Structure And Bonding.
From www.slideserve.com
PPT Metals PowerPoint Presentation, free download ID6413590 Why Are Metals Malleable In Terms Of Structure And Bonding The attractive forces between the metal ions. Metallic bonding is different from ionic and covalent bonding. The structure of metals explains their high melting and boiling points and their conductivity. Metals are malleable and ductile because of metallic bonding. The bonding that occurs in a metal is responsible for its distinctive properties: Metallic bonds are very strong so the giant. Why Are Metals Malleable In Terms Of Structure And Bonding.
From slideplayer.com
Chemical Bonding Chapter ppt download Why Are Metals Malleable In Terms Of Structure And Bonding The properties of a metal can be modified by mixing it with another substance to form an alloy. The bonding that occurs in a metal is responsible for its distinctive properties: Metallic bonding is it's own type of bond. The structure of metals explains their high melting and boiling points and their conductivity. Metallic bonds involve all of the metal. Why Are Metals Malleable In Terms Of Structure And Bonding.
From www.britannica.com
Metallic bond Properties, Examples, & Explanation Britannica Why Are Metals Malleable In Terms Of Structure And Bonding The bonding that occurs in a metal is responsible for its distinctive properties: Most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. Metallic bonding is it's own type of bond. Metallic bonding is different from ionic and covalent bonding. Metallic bonding & giant metallic lattice structures. The properties of a. Why Are Metals Malleable In Terms Of Structure And Bonding.
From slideplayer.com
Metallic bond. ppt download Why Are Metals Malleable In Terms Of Structure And Bonding Metallic bonding is different from ionic and covalent bonding. The attractive forces between the metal ions. The structure of metals explains their high melting and boiling points and their conductivity. Metallic bonding is it's own type of bond. The metallic bond is not fully broken until the metal boils. The properties of a metal can be modified by mixing it. Why Are Metals Malleable In Terms Of Structure And Bonding.
From www.slideserve.com
PPT Metals PowerPoint Presentation, free download ID1462875 Why Are Metals Malleable In Terms Of Structure And Bonding Metallic bonds are very strong so the giant metallic structure is strongly held together: The properties of a metal can be modified by mixing it with another substance to form an alloy. The bonding that occurs in a metal is responsible for its distinctive properties: Metallic bonding is different from ionic and covalent bonding. When a force is applied, the. Why Are Metals Malleable In Terms Of Structure And Bonding.
From chemnotcheem.com
Metallic bonding & giant metallic structure O Level Chemistry Notes Why Are Metals Malleable In Terms Of Structure And Bonding Metallic bonding & giant metallic lattice structures. The metallic bond is not fully broken until the metal boils. Most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. Metallic bonds are very strong so the giant metallic structure is strongly held together: Metallic bonding is it's own type of bond. In. Why Are Metals Malleable In Terms Of Structure And Bonding.
From studykaki.com
Explain, using structure and bonding, why alloys are stronger and Why Are Metals Malleable In Terms Of Structure And Bonding The properties of a metal can be modified by mixing it with another substance to form an alloy. In a molten metal, the metallic bond is still present, although the ordered structure has been broken down. Metallic bonding is it's own type of bond. Metallic bonds involve all of the metal atoms. The metallic bond is not fully broken until. Why Are Metals Malleable In Terms Of Structure And Bonding.
From slideplayer.com
Properties of Metallic Bonding ppt download Why Are Metals Malleable In Terms Of Structure And Bonding Luster, malleability, ductility, and excellent conductivity. In a molten metal, the metallic bond is still present, although the ordered structure has been broken down. Metallic bonding & giant metallic lattice structures. The properties of a metal can be modified by mixing it with another substance to form an alloy. The attractive forces between the metal ions. Metallic bonds involve all. Why Are Metals Malleable In Terms Of Structure And Bonding.
From www.slideshare.net
Metallic Bonding Why Are Metals Malleable In Terms Of Structure And Bonding Metallic bonds involve all of the metal atoms. Metals are malleable and ductile because of metallic bonding. The bonding that occurs in a metal is responsible for its distinctive properties: The properties of a metal can be modified by mixing it with another substance to form an alloy. Metallic bonding & giant metallic lattice structures. The structure of metals explains. Why Are Metals Malleable In Terms Of Structure And Bonding.
From www.thesciencehive.co.uk
Metallic Bonding (GCSE) — the science sauce Why Are Metals Malleable In Terms Of Structure And Bonding When a force is applied, the metal layers can slide. Metallic bonding is it's own type of bond. Most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. Metallic bonds are very strong so the giant metallic structure is strongly held together: The attractive forces between the metal ions. Metallic bonds. Why Are Metals Malleable In Terms Of Structure And Bonding.
From slideplayer.com
Metallic Bonds. ppt download Why Are Metals Malleable In Terms Of Structure And Bonding Metallic bonds involve all of the metal atoms. When a force is applied, the metal layers can slide. In a molten metal, the metallic bond is still present, although the ordered structure has been broken down. Metals are malleable and ductile because of metallic bonding. The structure of metals explains their high melting and boiling points and their conductivity. The. Why Are Metals Malleable In Terms Of Structure And Bonding.
From quizlet.com
Chemistry C2 Metallic Bonding Diagram Quizlet Why Are Metals Malleable In Terms Of Structure And Bonding The properties of a metal can be modified by mixing it with another substance to form an alloy. The metallic bond is not fully broken until the metal boils. Metallic bonding is it's own type of bond. Metals are malleable and ductile because of metallic bonding. Metallic bonding & giant metallic lattice structures. When a force is applied, the metal. Why Are Metals Malleable In Terms Of Structure And Bonding.
From slideplayer.com
Ionic & Metallic Bonding ppt download Why Are Metals Malleable In Terms Of Structure And Bonding Metallic bonding is it's own type of bond. Luster, malleability, ductility, and excellent conductivity. The attractive forces between the metal ions. Metallic bonding & giant metallic lattice structures. In a molten metal, the metallic bond is still present, although the ordered structure has been broken down. Metals are malleable and ductile because of metallic bonding. When a force is applied,. Why Are Metals Malleable In Terms Of Structure And Bonding.
From www.slideserve.com
PPT Objectives PowerPoint Presentation, free download ID5858440 Why Are Metals Malleable In Terms Of Structure And Bonding The metallic bond is not fully broken until the metal boils. Metals are malleable and ductile because of metallic bonding. The bonding that occurs in a metal is responsible for its distinctive properties: Luster, malleability, ductility, and excellent conductivity. In a molten metal, the metallic bond is still present, although the ordered structure has been broken down. The properties of. Why Are Metals Malleable In Terms Of Structure And Bonding.
From www.youtube.com
Metallic Bonding conductivity, malleability and high mp YouTube Why Are Metals Malleable In Terms Of Structure And Bonding Metallic bonds involve all of the metal atoms. Metallic bonding is different from ionic and covalent bonding. Metallic bonding & giant metallic lattice structures. The properties of a metal can be modified by mixing it with another substance to form an alloy. Metallic bonding is it's own type of bond. The structure of metals explains their high melting and boiling. Why Are Metals Malleable In Terms Of Structure And Bonding.
From www.expii.com
Metallic Bond — Formation & Compounds Expii Why Are Metals Malleable In Terms Of Structure And Bonding Metallic bonds involve all of the metal atoms. Metallic bonding & giant metallic lattice structures. Metallic bonding is it's own type of bond. Most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. In a molten metal, the metallic bond is still present, although the ordered structure has been broken down.. Why Are Metals Malleable In Terms Of Structure And Bonding.
From www.slideserve.com
PPT Chapter 7 “Metallic Bonding” PowerPoint Presentation, free Why Are Metals Malleable In Terms Of Structure And Bonding Metallic bonding is different from ionic and covalent bonding. When a force is applied, the metal layers can slide. Metallic bonding & giant metallic lattice structures. The metallic bond is not fully broken until the metal boils. Metals are malleable and ductile because of metallic bonding. The properties of a metal can be modified by mixing it with another substance. Why Are Metals Malleable In Terms Of Structure And Bonding.
From www.slideserve.com
PPT Bonding in Metals PowerPoint Presentation, free download ID4819941 Why Are Metals Malleable In Terms Of Structure And Bonding When a force is applied, the metal layers can slide. The structure of metals explains their high melting and boiling points and their conductivity. The bonding that occurs in a metal is responsible for its distinctive properties: In a molten metal, the metallic bond is still present, although the ordered structure has been broken down. The metallic bond is not. Why Are Metals Malleable In Terms Of Structure And Bonding.
From www.slideserve.com
PPT Structure and bonding in metals PowerPoint Presentation ID3178980 Why Are Metals Malleable In Terms Of Structure And Bonding When a force is applied, the metal layers can slide. The properties of a metal can be modified by mixing it with another substance to form an alloy. The bonding that occurs in a metal is responsible for its distinctive properties: Luster, malleability, ductility, and excellent conductivity. Metallic bonding is it's own type of bond. The structure of metals explains. Why Are Metals Malleable In Terms Of Structure And Bonding.
From www.slideserve.com
PPT Metallic bonding and properties PowerPoint Presentation ID3072363 Why Are Metals Malleable In Terms Of Structure And Bonding The metallic bond is not fully broken until the metal boils. Metallic bonds involve all of the metal atoms. When a force is applied, the metal layers can slide. Metallic bonds are very strong so the giant metallic structure is strongly held together: Metallic bonding is different from ionic and covalent bonding. Luster, malleability, ductility, and excellent conductivity. The properties. Why Are Metals Malleable In Terms Of Structure And Bonding.