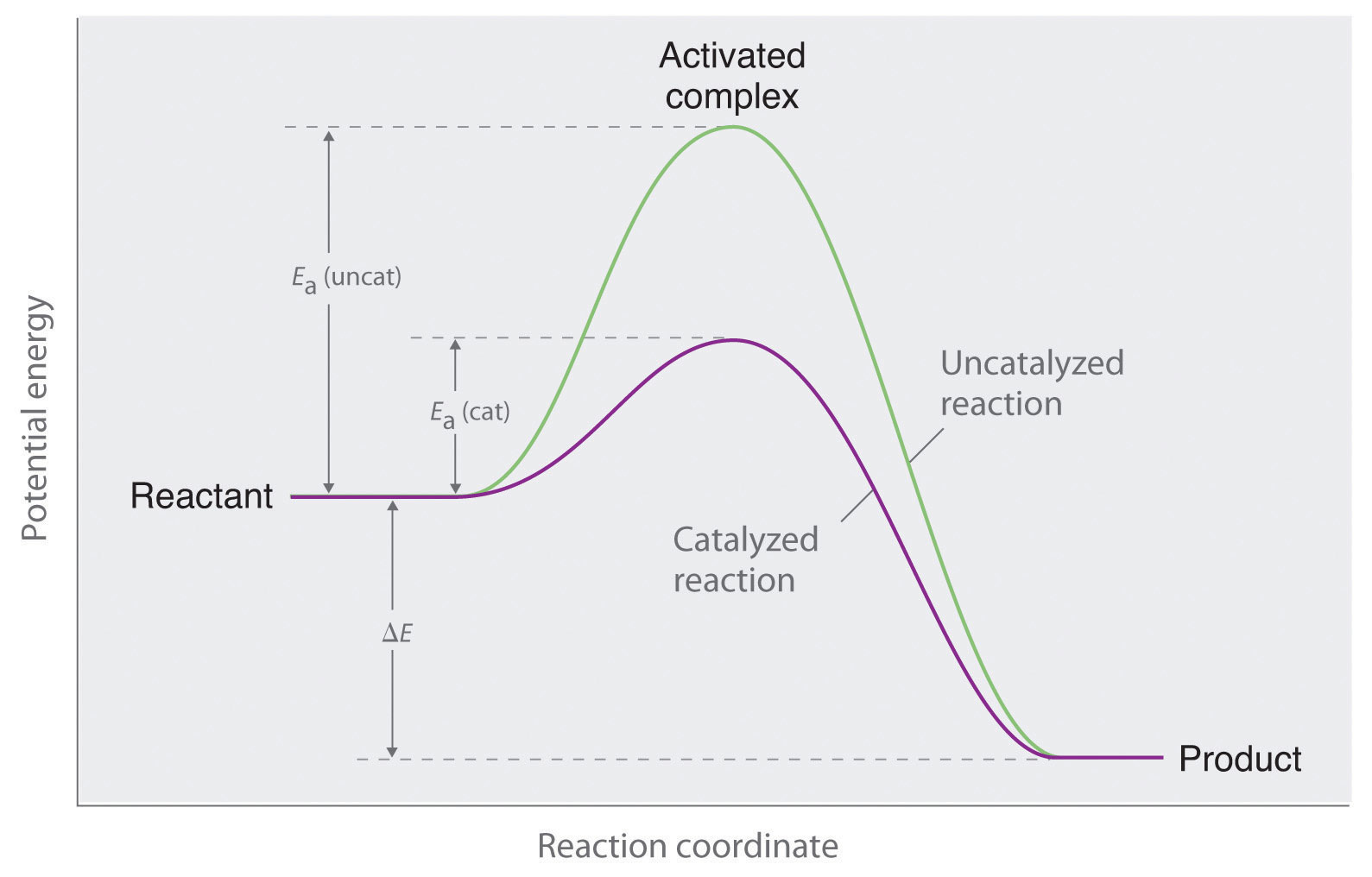
Catalysts Explained . A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is some material that speeds up chemical reactions. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. It interacts with the reactants in a cyclic manner promoting perhaps many reactions at the atomic or molecular level, but it is not consumed. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. This process is called catalysis. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself.
from 2012books.lardbucket.org
A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. A catalyst is some material that speeds up chemical reactions. It interacts with the reactants in a cyclic manner promoting perhaps many reactions at the atomic or molecular level, but it is not consumed. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. This process is called catalysis. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition.
Catalysis
Catalysts Explained A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. It interacts with the reactants in a cyclic manner promoting perhaps many reactions at the atomic or molecular level, but it is not consumed. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. This process is called catalysis. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst is some material that speeds up chemical reactions.
From www.thoughtco.com
Catalysis Definition in Chemistry Catalysts Explained With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. It interacts with the reactants in a cyclic manner promoting perhaps many reactions at the atomic or molecular level, but it is not consumed. A catalyst is some material that speeds up chemical reactions. A catalyst is a substance that. Catalysts Explained.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Catalysts Explained This process is called catalysis. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. It interacts with the reactants in a cyclic manner promoting perhaps many reactions at the atomic or molecular level, but it is not consumed. A catalyst is a substance that. Catalysts Explained.
From philschatz.com
Catalysis · Chemistry Catalysts Explained A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Catalyst, in chemistry,. Catalysts Explained.
From circuitdbplastered.z13.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalysts Explained A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. A catalyst is some material that speeds up chemical reactions. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is not consumed by the reaction and. Catalysts Explained.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalysts Explained This process is called catalysis. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to. Catalysts Explained.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalysts Explained Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A. Catalysts Explained.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalysts Explained A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts. Catalysts Explained.
From chemistryscore.com
Catalyst Learn Chemistry Online ChemistryScore Catalysts Explained With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being. Catalysts Explained.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog Catalysts Explained It interacts with the reactants in a cyclic manner promoting perhaps many reactions at the atomic or molecular level, but it is not consumed. This process is called catalysis. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. With a helping hand from a catalyst, molecules that might take years to. Catalysts Explained.
From courses.lumenlearning.com
12.7 Catalysis General College Chemistry II Catalysts Explained Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. A catalyst is some material that speeds up chemical reactions. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. It interacts with the reactants in a cyclic manner promoting perhaps many reactions at the atomic. Catalysts Explained.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2568751 Catalysts Explained A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Catalyst, in chemistry, any substance that increases the rate of a reaction. Catalysts Explained.
From www.youtube.com
Catalyst Affects Reaction Rate Energy Diagram with a Catalyst YouTube Catalysts Explained It interacts with the reactants in a cyclic manner promoting perhaps many reactions at the atomic or molecular level, but it is not consumed. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is some material that speeds up chemical reactions. This process is called catalysis. A catalyst is not consumed. Catalysts Explained.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID Catalysts Explained A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is some material that speeds up chemical reactions. A catalyst is not consumed by the reaction and. Catalysts Explained.
From 2012books.lardbucket.org
Catalysis Catalysts Explained Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. This process is called catalysis. A. Catalysts Explained.
From www.catalystseurope.org
What are catalysts? Catalysts Explained Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical. Catalysts Explained.
From schoolbag.info
A catalyst speeds up a reaction by providing the reactants with an Catalysts Explained Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. It interacts with the reactants in a cyclic manner promoting perhaps many reactions at the atomic or molecular level, but it is not consumed. With a. Catalysts Explained.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalysts Explained Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. A catalyst is some material that speeds up chemical reactions. Catalysts have no effect on the equilibrium constant and thus on. Catalysts Explained.
From www.smartkarrot.com
Catalyst Explained 3 SaaS Business Catalysts That Can Change the Game Catalysts Explained A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. It. Catalysts Explained.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Catalysts Explained A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. A catalyst is some material that speeds up chemical reactions. Catalyst, in chemistry, any substance. Catalysts Explained.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts Catalysts Explained A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. It. Catalysts Explained.
From www.slideserve.com
PPT Mechanisms, Catalysts Intermediates and k PowerPoint Presentation Catalysts Explained A catalyst is some material that speeds up chemical reactions. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. It interacts with the reactants in a cyclic manner. Catalysts Explained.
From www.youtube.com
How does a CATALYST work ? YouTube Catalysts Explained With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. A catalyst is some material that speeds up chemical reactions. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or. Catalysts Explained.
From slidetodoc.com
ENZYME BIOLOGICAL CATALYST Enzyme As Catalyst All enzymes Catalysts Explained With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching. Catalysts Explained.
From www.slideserve.com
PPT Homogeneous Catalysis Introduction PowerPoint Presentation Catalysts Explained A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. It interacts with the reactants in a cyclic manner promoting perhaps many reactions at the atomic or molecular level, but it is not consumed. Catalyst, in chemistry, any substance that increases the rate of a reaction without. Catalysts Explained.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub Catalysts Explained This process is called catalysis. A catalyst is some material that speeds up chemical reactions. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed.. Catalysts Explained.
From design1systems.com
The Impact of Catalysts on Reaction Coordinate Diagrams Explained Catalysts Explained Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. A catalyst is. Catalysts Explained.
From www.youtube.com
Catalytic Converter Working Principle 2 way and 3 way, Function of Catalysts Explained Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst is another substance. Catalysts Explained.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalysts Explained With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. This process is called catalysis. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. A catalyst is. Catalysts Explained.
From www.waca.msf.org
Lesson Explainer Catalysts, Catalyst Catalysts Explained With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. This process is called catalysis. It interacts with the reactants in a cyclic manner promoting perhaps many reactions at the atomic or molecular level, but it is not consumed. Catalyst, in chemistry, any substance that increases the rate of a. Catalysts Explained.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalysts Explained It interacts with the reactants in a cyclic manner promoting perhaps many reactions at the atomic or molecular level, but it is not consumed. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. A catalyst is a substance that speeds up a chemical reaction,. Catalysts Explained.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica Catalysts Explained Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. A catalyst is. Catalysts Explained.
From www.savemyexams.co.uk
Catalysts (1.7.6) AQA A Level Chemistry Revision Notes 2017 Save My Catalysts Explained This process is called catalysis. It interacts with the reactants in a cyclic manner promoting perhaps many reactions at the atomic or molecular level, but it is not consumed. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. A catalyst is some material that speeds up chemical reactions. A catalyst is. Catalysts Explained.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalysts Explained A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. A catalyst is some material that speeds up chemical reactions. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. With a helping hand from a catalyst,. Catalysts Explained.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalysts Explained A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. With a helping hand from a catalyst, molecules that might take years. Catalysts Explained.
From www.researchgate.net
Classification and summary of different catalysts. Download Catalysts Explained Catalysts have no effect on the equilibrium constant and thus on the equilibrium composition. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. A catalyst is some material that speeds up chemical reactions. It interacts with the reactants in a cyclic manner promoting perhaps many reactions at the atomic or molecular. Catalysts Explained.