Dietary Supplement Fda Definition . Ingredient means any substance that is used in the manufacture of a dietary supplement and that is intended to be present. The term, dietary supplement, was defined by the dietary supplement health and education act (dshea) as a product (other than tobacco) that. Learn how consumers, health care providers, and others can report a complaint, concern, or problem related to dietary. They are not medicines and are not intended to treat, diagnose,. Fda is the federal agency that oversees both supplements and medicines, but fda regulations for dietary supplements are different from. Federal regulation of dietary supplements dietary supplements are products intended to supplement the diet. Food and drug administration (fda) requires specific safety information from a manufacturer intending to market a dietary. Generally, to the extent a. Dietary supplements are intended to add to or supplement the diet and are different from conventional food.
from www.artworkflowhq.com
Generally, to the extent a. Federal regulation of dietary supplements dietary supplements are products intended to supplement the diet. Ingredient means any substance that is used in the manufacture of a dietary supplement and that is intended to be present. The term, dietary supplement, was defined by the dietary supplement health and education act (dshea) as a product (other than tobacco) that. Fda is the federal agency that oversees both supplements and medicines, but fda regulations for dietary supplements are different from. Learn how consumers, health care providers, and others can report a complaint, concern, or problem related to dietary. Dietary supplements are intended to add to or supplement the diet and are different from conventional food. They are not medicines and are not intended to treat, diagnose,. Food and drug administration (fda) requires specific safety information from a manufacturer intending to market a dietary.
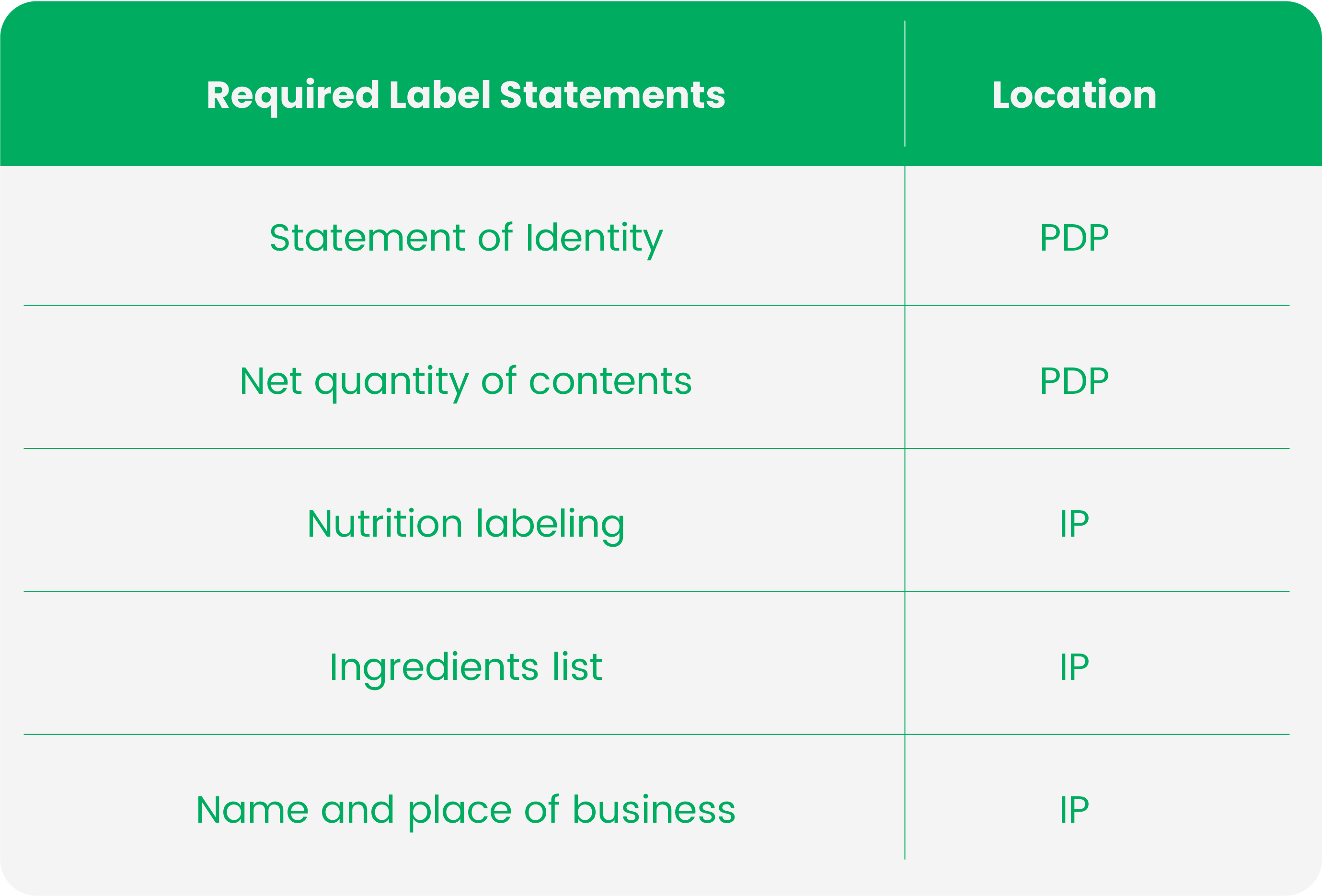
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Supplements
Dietary Supplement Fda Definition Food and drug administration (fda) requires specific safety information from a manufacturer intending to market a dietary. Ingredient means any substance that is used in the manufacture of a dietary supplement and that is intended to be present. Federal regulation of dietary supplements dietary supplements are products intended to supplement the diet. Dietary supplements are intended to add to or supplement the diet and are different from conventional food. Generally, to the extent a. Food and drug administration (fda) requires specific safety information from a manufacturer intending to market a dietary. Learn how consumers, health care providers, and others can report a complaint, concern, or problem related to dietary. They are not medicines and are not intended to treat, diagnose,. The term, dietary supplement, was defined by the dietary supplement health and education act (dshea) as a product (other than tobacco) that. Fda is the federal agency that oversees both supplements and medicines, but fda regulations for dietary supplements are different from.
From www.slideserve.com
PPT FDA Regulation of Product Claims PowerPoint Presentation, free Dietary Supplement Fda Definition Generally, to the extent a. Learn how consumers, health care providers, and others can report a complaint, concern, or problem related to dietary. Food and drug administration (fda) requires specific safety information from a manufacturer intending to market a dietary. The term, dietary supplement, was defined by the dietary supplement health and education act (dshea) as a product (other than. Dietary Supplement Fda Definition.
From nutrition.ftempo.com
Fda Nutrition Label Guidelines 2017 Nutrition Ftempo Dietary Supplement Fda Definition Generally, to the extent a. Dietary supplements are intended to add to or supplement the diet and are different from conventional food. The term, dietary supplement, was defined by the dietary supplement health and education act (dshea) as a product (other than tobacco) that. Food and drug administration (fda) requires specific safety information from a manufacturer intending to market a. Dietary Supplement Fda Definition.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Supplements Dietary Supplement Fda Definition Generally, to the extent a. Dietary supplements are intended to add to or supplement the diet and are different from conventional food. The term, dietary supplement, was defined by the dietary supplement health and education act (dshea) as a product (other than tobacco) that. They are not medicines and are not intended to treat, diagnose,. Food and drug administration (fda). Dietary Supplement Fda Definition.
From studylib.net
FDA Product Data Sheet Dietary Supplements Dietary Supplement Fda Definition Generally, to the extent a. Fda is the federal agency that oversees both supplements and medicines, but fda regulations for dietary supplements are different from. They are not medicines and are not intended to treat, diagnose,. Federal regulation of dietary supplements dietary supplements are products intended to supplement the diet. Dietary supplements are intended to add to or supplement the. Dietary Supplement Fda Definition.
From theherbalacademy.com
What FDA Dietary Supplement Regulations Mean For Herbalists Dietary Supplement Fda Definition Generally, to the extent a. The term, dietary supplement, was defined by the dietary supplement health and education act (dshea) as a product (other than tobacco) that. Food and drug administration (fda) requires specific safety information from a manufacturer intending to market a dietary. Fda is the federal agency that oversees both supplements and medicines, but fda regulations for dietary. Dietary Supplement Fda Definition.
From www.scribd.com
Dietary Supplement Quality Guide PDF Dietary Supplements Food And Dietary Supplement Fda Definition They are not medicines and are not intended to treat, diagnose,. Learn how consumers, health care providers, and others can report a complaint, concern, or problem related to dietary. Federal regulation of dietary supplements dietary supplements are products intended to supplement the diet. Food and drug administration (fda) requires specific safety information from a manufacturer intending to market a dietary.. Dietary Supplement Fda Definition.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Dietary Supplement Fda Definition Federal regulation of dietary supplements dietary supplements are products intended to supplement the diet. Food and drug administration (fda) requires specific safety information from a manufacturer intending to market a dietary. Ingredient means any substance that is used in the manufacture of a dietary supplement and that is intended to be present. The term, dietary supplement, was defined by the. Dietary Supplement Fda Definition.
From synthys-medical.com
FDA Dietary Supplements SYNTHYS MEDICAL Dietary Supplement Fda Definition They are not medicines and are not intended to treat, diagnose,. Fda is the federal agency that oversees both supplements and medicines, but fda regulations for dietary supplements are different from. Dietary supplements are intended to add to or supplement the diet and are different from conventional food. Learn how consumers, health care providers, and others can report a complaint,. Dietary Supplement Fda Definition.
From www.slideserve.com
PPT Dietary Supplements Kava a case study PowerPoint Presentation Dietary Supplement Fda Definition Dietary supplements are intended to add to or supplement the diet and are different from conventional food. Generally, to the extent a. Food and drug administration (fda) requires specific safety information from a manufacturer intending to market a dietary. Federal regulation of dietary supplements dietary supplements are products intended to supplement the diet. They are not medicines and are not. Dietary Supplement Fda Definition.
From www.slideserve.com
PPT PRESENTATION ON Supplementary FOOD PRODUCTS PowerPoint Dietary Supplement Fda Definition Federal regulation of dietary supplements dietary supplements are products intended to supplement the diet. Ingredient means any substance that is used in the manufacture of a dietary supplement and that is intended to be present. Fda is the federal agency that oversees both supplements and medicines, but fda regulations for dietary supplements are different from. The term, dietary supplement, was. Dietary Supplement Fda Definition.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Dietary Supplement Fda Definition Food and drug administration (fda) requires specific safety information from a manufacturer intending to market a dietary. Dietary supplements are intended to add to or supplement the diet and are different from conventional food. Fda is the federal agency that oversees both supplements and medicines, but fda regulations for dietary supplements are different from. They are not medicines and are. Dietary Supplement Fda Definition.
From ar.inspiredpencil.com
Dietary Supplements Label Dietary Supplement Fda Definition Fda is the federal agency that oversees both supplements and medicines, but fda regulations for dietary supplements are different from. They are not medicines and are not intended to treat, diagnose,. Dietary supplements are intended to add to or supplement the diet and are different from conventional food. Federal regulation of dietary supplements dietary supplements are products intended to supplement. Dietary Supplement Fda Definition.
From www.chameleon-pharma.com
Food Supplements regulations USA Chameleon Pharma Consulting Dietary Supplement Fda Definition Learn how consumers, health care providers, and others can report a complaint, concern, or problem related to dietary. Ingredient means any substance that is used in the manufacture of a dietary supplement and that is intended to be present. Dietary supplements are intended to add to or supplement the diet and are different from conventional food. They are not medicines. Dietary Supplement Fda Definition.
From www.slideshare.net
Dietary supplements Dietary Supplement Fda Definition Learn how consumers, health care providers, and others can report a complaint, concern, or problem related to dietary. Generally, to the extent a. They are not medicines and are not intended to treat, diagnose,. Ingredient means any substance that is used in the manufacture of a dietary supplement and that is intended to be present. The term, dietary supplement, was. Dietary Supplement Fda Definition.
From www.nutraingredients-usa.com
FDA provides industry guidance for nutrient conversion units on Dietary Supplement Fda Definition Learn how consumers, health care providers, and others can report a complaint, concern, or problem related to dietary. Ingredient means any substance that is used in the manufacture of a dietary supplement and that is intended to be present. They are not medicines and are not intended to treat, diagnose,. Dietary supplements are intended to add to or supplement the. Dietary Supplement Fda Definition.
From www.slideserve.com
PPT Dietary Supplements PowerPoint Presentation, free download ID Dietary Supplement Fda Definition They are not medicines and are not intended to treat, diagnose,. Federal regulation of dietary supplements dietary supplements are products intended to supplement the diet. Learn how consumers, health care providers, and others can report a complaint, concern, or problem related to dietary. Food and drug administration (fda) requires specific safety information from a manufacturer intending to market a dietary.. Dietary Supplement Fda Definition.
From www.slideserve.com
PPT Defining Dietary Supplements PowerPoint Presentation, free Dietary Supplement Fda Definition Food and drug administration (fda) requires specific safety information from a manufacturer intending to market a dietary. Ingredient means any substance that is used in the manufacture of a dietary supplement and that is intended to be present. Fda is the federal agency that oversees both supplements and medicines, but fda regulations for dietary supplements are different from. They are. Dietary Supplement Fda Definition.
From www.slideserve.com
PPT Chapter 107 PowerPoint Presentation, free download ID3088867 Dietary Supplement Fda Definition The term, dietary supplement, was defined by the dietary supplement health and education act (dshea) as a product (other than tobacco) that. They are not medicines and are not intended to treat, diagnose,. Food and drug administration (fda) requires specific safety information from a manufacturer intending to market a dietary. Generally, to the extent a. Dietary supplements are intended to. Dietary Supplement Fda Definition.
From www.dailyintakeblog.com
FDA Confirms NacetylLcysteine (NAC) is Excluded From the Dietary Dietary Supplement Fda Definition They are not medicines and are not intended to treat, diagnose,. Dietary supplements are intended to add to or supplement the diet and are different from conventional food. Federal regulation of dietary supplements dietary supplements are products intended to supplement the diet. The term, dietary supplement, was defined by the dietary supplement health and education act (dshea) as a product. Dietary Supplement Fda Definition.
From cohenhealthcarelaw.com
FDA Dietary Supplement Labeling Requirements Comply or Die Cohen Dietary Supplement Fda Definition Dietary supplements are intended to add to or supplement the diet and are different from conventional food. Federal regulation of dietary supplements dietary supplements are products intended to supplement the diet. Food and drug administration (fda) requires specific safety information from a manufacturer intending to market a dietary. Learn how consumers, health care providers, and others can report a complaint,. Dietary Supplement Fda Definition.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Supplements Dietary Supplement Fda Definition Food and drug administration (fda) requires specific safety information from a manufacturer intending to market a dietary. Fda is the federal agency that oversees both supplements and medicines, but fda regulations for dietary supplements are different from. Federal regulation of dietary supplements dietary supplements are products intended to supplement the diet. Ingredient means any substance that is used in the. Dietary Supplement Fda Definition.
From www.dreamstime.com
FDA Nutrition Supplement Facts Labeling Labels a Proprietary Blend of Dietary Supplement Fda Definition Generally, to the extent a. Fda is the federal agency that oversees both supplements and medicines, but fda regulations for dietary supplements are different from. Federal regulation of dietary supplements dietary supplements are products intended to supplement the diet. Food and drug administration (fda) requires specific safety information from a manufacturer intending to market a dietary. Learn how consumers, health. Dietary Supplement Fda Definition.
From www.arnoldporter.com
FDA Gives Nutrition Facts and Supplement Facts Labels a Major Makeover Dietary Supplement Fda Definition Food and drug administration (fda) requires specific safety information from a manufacturer intending to market a dietary. Fda is the federal agency that oversees both supplements and medicines, but fda regulations for dietary supplements are different from. Generally, to the extent a. Dietary supplements are intended to add to or supplement the diet and are different from conventional food. Ingredient. Dietary Supplement Fda Definition.
From www.fdalisting.com
U.S. FDA Food, Beverage and Dietary Supplement Labeling Requirements Dietary Supplement Fda Definition Ingredient means any substance that is used in the manufacture of a dietary supplement and that is intended to be present. They are not medicines and are not intended to treat, diagnose,. Learn how consumers, health care providers, and others can report a complaint, concern, or problem related to dietary. Fda is the federal agency that oversees both supplements and. Dietary Supplement Fda Definition.
From www.onlinelabels.com
What You Need to Know About the New FDA Nutrition Fact Label Dietary Supplement Fda Definition Dietary supplements are intended to add to or supplement the diet and are different from conventional food. They are not medicines and are not intended to treat, diagnose,. Generally, to the extent a. Federal regulation of dietary supplements dietary supplements are products intended to supplement the diet. Fda is the federal agency that oversees both supplements and medicines, but fda. Dietary Supplement Fda Definition.
From www.slideshare.net
Dietary supplements Dietary Supplement Fda Definition The term, dietary supplement, was defined by the dietary supplement health and education act (dshea) as a product (other than tobacco) that. Ingredient means any substance that is used in the manufacture of a dietary supplement and that is intended to be present. Dietary supplements are intended to add to or supplement the diet and are different from conventional food.. Dietary Supplement Fda Definition.
From www.slideserve.com
PPT Dietary Supplements Kava a case study PowerPoint Presentation Dietary Supplement Fda Definition Fda is the federal agency that oversees both supplements and medicines, but fda regulations for dietary supplements are different from. Food and drug administration (fda) requires specific safety information from a manufacturer intending to market a dietary. Dietary supplements are intended to add to or supplement the diet and are different from conventional food. They are not medicines and are. Dietary Supplement Fda Definition.
From www.alamy.com
FDA 101 Dietary Supplements Stock Photo Alamy Dietary Supplement Fda Definition Dietary supplements are intended to add to or supplement the diet and are different from conventional food. Federal regulation of dietary supplements dietary supplements are products intended to supplement the diet. They are not medicines and are not intended to treat, diagnose,. Food and drug administration (fda) requires specific safety information from a manufacturer intending to market a dietary. The. Dietary Supplement Fda Definition.
From www.dreamstime.com
FDA Nutrition Supplement Facts Labeling Labels Dietary Supplement Dietary Supplement Fda Definition The term, dietary supplement, was defined by the dietary supplement health and education act (dshea) as a product (other than tobacco) that. Learn how consumers, health care providers, and others can report a complaint, concern, or problem related to dietary. Federal regulation of dietary supplements dietary supplements are products intended to supplement the diet. Fda is the federal agency that. Dietary Supplement Fda Definition.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Dietary Supplement Fda Definition They are not medicines and are not intended to treat, diagnose,. Dietary supplements are intended to add to or supplement the diet and are different from conventional food. Fda is the federal agency that oversees both supplements and medicines, but fda regulations for dietary supplements are different from. Generally, to the extent a. Food and drug administration (fda) requires specific. Dietary Supplement Fda Definition.
From www.slideserve.com
PPT FDA Dietary Supplement Update PowerPoint Presentation, free Dietary Supplement Fda Definition The term, dietary supplement, was defined by the dietary supplement health and education act (dshea) as a product (other than tobacco) that. Dietary supplements are intended to add to or supplement the diet and are different from conventional food. Fda is the federal agency that oversees both supplements and medicines, but fda regulations for dietary supplements are different from. Generally,. Dietary Supplement Fda Definition.
From www.slideserve.com
PPT Dietary Supplements PowerPoint Presentation, free download ID Dietary Supplement Fda Definition Fda is the federal agency that oversees both supplements and medicines, but fda regulations for dietary supplements are different from. Ingredient means any substance that is used in the manufacture of a dietary supplement and that is intended to be present. They are not medicines and are not intended to treat, diagnose,. Learn how consumers, health care providers, and others. Dietary Supplement Fda Definition.
From www.supplements.education
Understanding the FDA's Classification of Dietary Supplements Dietary Supplement Fda Definition Fda is the federal agency that oversees both supplements and medicines, but fda regulations for dietary supplements are different from. Federal regulation of dietary supplements dietary supplements are products intended to supplement the diet. Dietary supplements are intended to add to or supplement the diet and are different from conventional food. Learn how consumers, health care providers, and others can. Dietary Supplement Fda Definition.
From www.fdalisting.com
U.S. FDA Food, Beverage and Dietary Supplement Labeling Requirements Dietary Supplement Fda Definition Ingredient means any substance that is used in the manufacture of a dietary supplement and that is intended to be present. Generally, to the extent a. Federal regulation of dietary supplements dietary supplements are products intended to supplement the diet. Food and drug administration (fda) requires specific safety information from a manufacturer intending to market a dietary. They are not. Dietary Supplement Fda Definition.
From www.youtube.com
How Does the FDA Regulate Dietary Supplements? YouTube Dietary Supplement Fda Definition Fda is the federal agency that oversees both supplements and medicines, but fda regulations for dietary supplements are different from. Dietary supplements are intended to add to or supplement the diet and are different from conventional food. Federal regulation of dietary supplements dietary supplements are products intended to supplement the diet. Food and drug administration (fda) requires specific safety information. Dietary Supplement Fda Definition.