Bromide To Fluoride . Swarts reaction (also known as halogen exchange reaction or swarts fluorination reaction) is a procedure that converts alkyl chloride or alkyl bromide to alkyl fluoride. This is accomplished by heating the alkyl chloride/bromide in the presence of a heavy metal fluoride (for example, silver fluoride or mercurous fluoride). There are two different types of reaction which might go on when concentrated sulphuric acid is added to a solid ionic halide like sodium fluoride, chloride, bromide or iodide. There is one cobalt ion. This is done by heating of the alkyl. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. Swarts’ reaction is generally used to get alkyl fluorides from alkyl chlorides or alkyl bromides. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. Only one ion of each is needed.
from www.chegg.com
There is one cobalt ion. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. This is done by heating of the alkyl. Swarts’ reaction is generally used to get alkyl fluorides from alkyl chlorides or alkyl bromides. This is accomplished by heating the alkyl chloride/bromide in the presence of a heavy metal fluoride (for example, silver fluoride or mercurous fluoride). If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. There are two different types of reaction which might go on when concentrated sulphuric acid is added to a solid ionic halide like sodium fluoride, chloride, bromide or iodide. Only one ion of each is needed. Swarts reaction (also known as halogen exchange reaction or swarts fluorination reaction) is a procedure that converts alkyl chloride or alkyl bromide to alkyl fluoride.
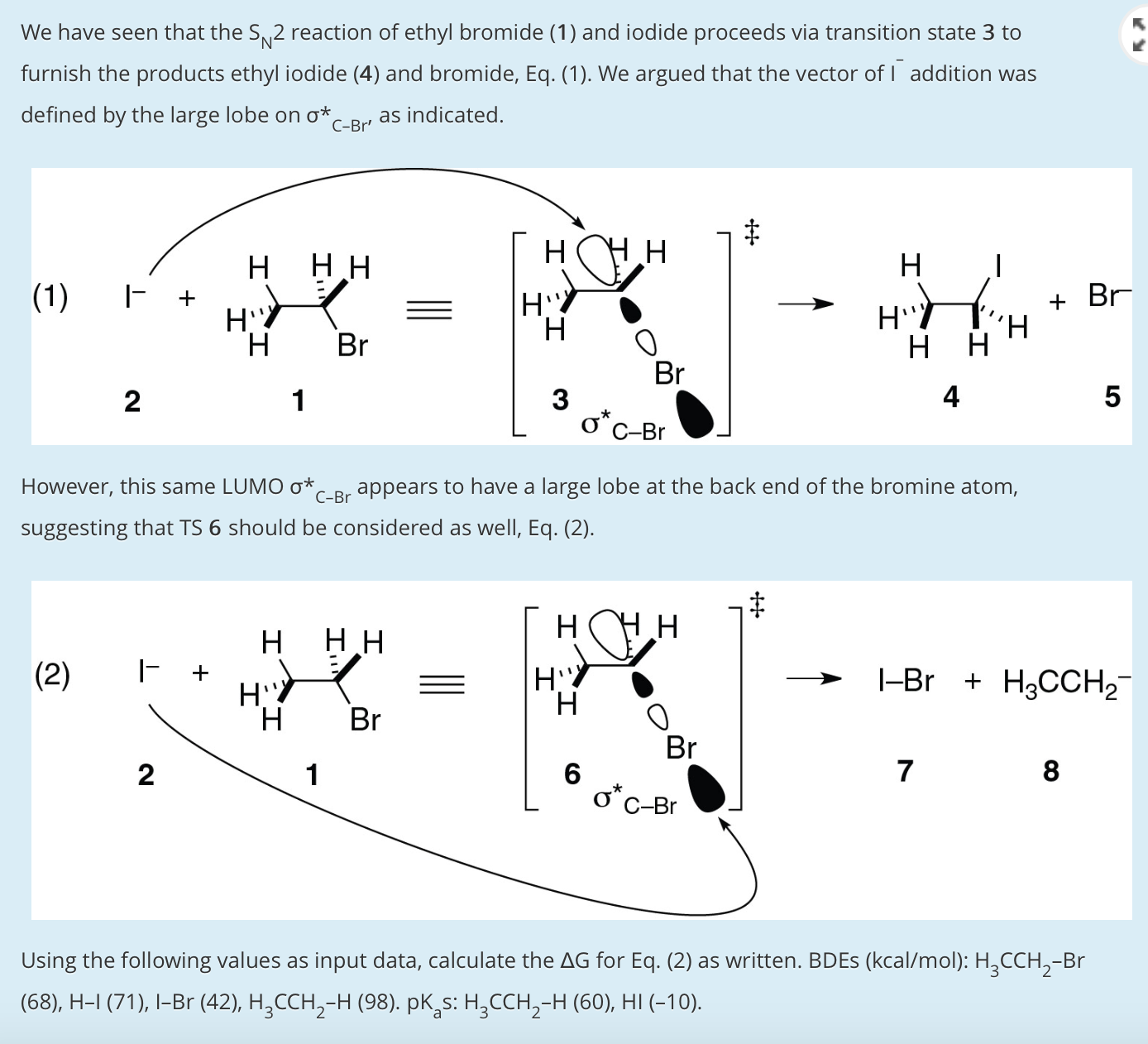
Solved We have seen that the SN2 reaction of ethyl bromide
Bromide To Fluoride This is accomplished by heating the alkyl chloride/bromide in the presence of a heavy metal fluoride (for example, silver fluoride or mercurous fluoride). If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. Swarts reaction (also known as halogen exchange reaction or swarts fluorination reaction) is a procedure that converts alkyl chloride or alkyl bromide to alkyl fluoride. This is done by heating of the alkyl. There are two different types of reaction which might go on when concentrated sulphuric acid is added to a solid ionic halide like sodium fluoride, chloride, bromide or iodide. Only one ion of each is needed. This is accomplished by heating the alkyl chloride/bromide in the presence of a heavy metal fluoride (for example, silver fluoride or mercurous fluoride). If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. Swarts’ reaction is generally used to get alkyl fluorides from alkyl chlorides or alkyl bromides. There is one cobalt ion.
From byjus.com
Write the molecular formulae for the compound Copper II Bromide Bromide To Fluoride If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. Swarts reaction (also known as halogen exchange reaction or swarts fluorination reaction) is a procedure that converts alkyl chloride or alkyl bromide to alkyl fluoride. Only one ion. Bromide To Fluoride.
From byjus.com
Phenyl magnesium bromide reacts with methanol to give Bromide To Fluoride If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. Swarts’ reaction is generally used to get alkyl fluorides from alkyl chlorides or alkyl bromides. This is done by heating of the alkyl. There is one cobalt ion.. Bromide To Fluoride.
From www.rpicorp.com
E718001.0 Ethidium Bromide, Powder, 1 Gram Bromide To Fluoride This is done by heating of the alkyl. Swarts reaction (also known as halogen exchange reaction or swarts fluorination reaction) is a procedure that converts alkyl chloride or alkyl bromide to alkyl fluoride. Swarts’ reaction is generally used to get alkyl fluorides from alkyl chlorides or alkyl bromides. There are two different types of reaction which might go on when. Bromide To Fluoride.
From www.indiamart.com
BioTech Grade Powder Sodium Bromide for Petroleum Industry, for Bromide To Fluoride If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. This is accomplished by heating the alkyl chloride/bromide in the presence of a heavy metal fluoride (for example, silver fluoride or mercurous fluoride). If we look at the ionic compound consisting of. Bromide To Fluoride.
From testbook.com
Lithium Bromide Learn Structure, Properties, Preparation & Uses. Bromide To Fluoride There is one cobalt ion. Only one ion of each is needed. This is done by heating of the alkyl. Swarts reaction (also known as halogen exchange reaction or swarts fluorination reaction) is a procedure that converts alkyl chloride or alkyl bromide to alkyl fluoride. Swarts’ reaction is generally used to get alkyl fluorides from alkyl chlorides or alkyl bromides.. Bromide To Fluoride.
From www.aluminummanufacturers.org
Aluminum Bromide Aluminum Sulfate Aluminum Manufacturers Bromide To Fluoride There is one cobalt ion. Only one ion of each is needed. This is done by heating of the alkyl. This is accomplished by heating the alkyl chloride/bromide in the presence of a heavy metal fluoride (for example, silver fluoride or mercurous fluoride). If we look at the ionic compound consisting of lithium ions and bromide ions, we see that. Bromide To Fluoride.
From sielc.com
Vinyl bromide SIELC Technologies Bromide To Fluoride If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. This is accomplished by heating the alkyl chloride/bromide in the presence of a heavy metal fluoride (for example, silver fluoride or mercurous fluoride). Only one ion of each. Bromide To Fluoride.
From www.chemistryworld.com
Potassium bromide Podcast Chemistry World Bromide To Fluoride Only one ion of each is needed. This is accomplished by heating the alkyl chloride/bromide in the presence of a heavy metal fluoride (for example, silver fluoride or mercurous fluoride). Swarts’ reaction is generally used to get alkyl fluorides from alkyl chlorides or alkyl bromides. If we look at the ionic compound consisting of lithium ions and bromide ions, we. Bromide To Fluoride.
From www.macsenlab.com
Pinaverium Bromide API 53251948 Manufacturer & Supplier Bromide To Fluoride This is accomplished by heating the alkyl chloride/bromide in the presence of a heavy metal fluoride (for example, silver fluoride or mercurous fluoride). Swarts reaction (also known as halogen exchange reaction or swarts fluorination reaction) is a procedure that converts alkyl chloride or alkyl bromide to alkyl fluoride. Only one ion of each is needed. There are two different types. Bromide To Fluoride.
From www.bhphotovideo.com
Photographers' Formulary Sodium Bromide 1 Lb. 101180 1LB B&H Bromide To Fluoride Only one ion of each is needed. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. Swarts’ reaction is generally used to get alkyl fluorides from alkyl chlorides or alkyl bromides. Swarts reaction (also known as halogen exchange reaction or swarts. Bromide To Fluoride.
From www.indiamart.com
Sodium Bromide 45, Bromide salt of sodium, NaBr, 7647156, Sedoneural Bromide To Fluoride Swarts reaction (also known as halogen exchange reaction or swarts fluorination reaction) is a procedure that converts alkyl chloride or alkyl bromide to alkyl fluoride. There are two different types of reaction which might go on when concentrated sulphuric acid is added to a solid ionic halide like sodium fluoride, chloride, bromide or iodide. There is one cobalt ion. This. Bromide To Fluoride.
From www.rpicorp.com
E718005.0 Ethidium Bromide, Powder, 5 Grams Bromide To Fluoride There is one cobalt ion. This is done by heating of the alkyl. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. This is accomplished by heating the alkyl chloride/bromide in the presence of a heavy metal fluoride (for example, silver. Bromide To Fluoride.
From www.chegg.com
Solved We have seen that the SN2 reaction of ethyl bromide Bromide To Fluoride Swarts reaction (also known as halogen exchange reaction or swarts fluorination reaction) is a procedure that converts alkyl chloride or alkyl bromide to alkyl fluoride. There are two different types of reaction which might go on when concentrated sulphuric acid is added to a solid ionic halide like sodium fluoride, chloride, bromide or iodide. Only one ion of each is. Bromide To Fluoride.
From byjus.com
Mechanism of Hoffman bromide degradation reaction Bromide To Fluoride Swarts reaction (also known as halogen exchange reaction or swarts fluorination reaction) is a procedure that converts alkyl chloride or alkyl bromide to alkyl fluoride. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. This is accomplished. Bromide To Fluoride.
From www.nanochemazone.com
1Dodecyl3methylimidazolium Bromide Low Price 45 High Purity Bromide To Fluoride If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. This is accomplished by heating the alkyl chloride/bromide in the presence of a heavy metal fluoride (for example, silver fluoride or mercurous fluoride). Swarts reaction (also known as halogen exchange reaction or. Bromide To Fluoride.
From us.metoree.com
41 Ethyl Bromide Manufacturers in 2024 Metoree Bromide To Fluoride Swarts’ reaction is generally used to get alkyl fluorides from alkyl chlorides or alkyl bromides. There is one cobalt ion. This is accomplished by heating the alkyl chloride/bromide in the presence of a heavy metal fluoride (for example, silver fluoride or mercurous fluoride). If we look at the ionic compound consisting of lithium ions and bromide ions, we see that. Bromide To Fluoride.
From www.chegg.com
Solved ammonium bromide calcium fluoride The pH will be less Bromide To Fluoride There are two different types of reaction which might go on when concentrated sulphuric acid is added to a solid ionic halide like sodium fluoride, chloride, bromide or iodide. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. There is one. Bromide To Fluoride.
From www.indiamart.com
NCETYLN,N,N TRIMETHYL AMMONIUM BROMIDE 500g, 98, 500gm Bottle at Rs Bromide To Fluoride This is done by heating of the alkyl. This is accomplished by heating the alkyl chloride/bromide in the presence of a heavy metal fluoride (for example, silver fluoride or mercurous fluoride). Swarts reaction (also known as halogen exchange reaction or swarts fluorination reaction) is a procedure that converts alkyl chloride or alkyl bromide to alkyl fluoride. Only one ion of. Bromide To Fluoride.
From hamptonresearch.com
Hampton Research Bromide To Fluoride If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. Only one ion of each is needed. This is accomplished by heating the alkyl chloride/bromide in the presence of a heavy metal fluoride (for example, silver fluoride or. Bromide To Fluoride.
From byjus.com
Briefly explain the process of electrolysis of molten Lead Bromide Bromide To Fluoride There are two different types of reaction which might go on when concentrated sulphuric acid is added to a solid ionic halide like sodium fluoride, chloride, bromide or iodide. This is accomplished by heating the alkyl chloride/bromide in the presence of a heavy metal fluoride (for example, silver fluoride or mercurous fluoride). Swarts’ reaction is generally used to get alkyl. Bromide To Fluoride.
From www.dreamstime.com
3D Image of Vinyl Bromide Skeletal Formula Stock Illustration Bromide To Fluoride This is accomplished by heating the alkyl chloride/bromide in the presence of a heavy metal fluoride (for example, silver fluoride or mercurous fluoride). This is done by heating of the alkyl. There are two different types of reaction which might go on when concentrated sulphuric acid is added to a solid ionic halide like sodium fluoride, chloride, bromide or iodide.. Bromide To Fluoride.
From www.shutterstock.com
Sodium Bromide Properties Chemical Compound Structure Stock Vector Bromide To Fluoride If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. There is one cobalt ion. Only one ion of each is needed. This is accomplished by heating the alkyl chloride/bromide in the presence of a heavy metal fluoride. Bromide To Fluoride.
From www.dreamstime.com
Potassium Bromide Chemical Formula on Waterdrop Background Stock Bromide To Fluoride Swarts reaction (also known as halogen exchange reaction or swarts fluorination reaction) is a procedure that converts alkyl chloride or alkyl bromide to alkyl fluoride. Swarts’ reaction is generally used to get alkyl fluorides from alkyl chlorides or alkyl bromides. There are two different types of reaction which might go on when concentrated sulphuric acid is added to a solid. Bromide To Fluoride.
From www.solcohealthcare.com
Rocuronium Bromide Injection Solco Healthcare Bromide To Fluoride Only one ion of each is needed. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and. Bromide To Fluoride.
From www.nanochemazone.com
1Butyl1methylpyrrolidinium Bromide Low Price 40 High Purity Bromide To Fluoride There is one cobalt ion. Swarts reaction (also known as halogen exchange reaction or swarts fluorination reaction) is a procedure that converts alkyl chloride or alkyl bromide to alkyl fluoride. This is accomplished by heating the alkyl chloride/bromide in the presence of a heavy metal fluoride (for example, silver fluoride or mercurous fluoride). Swarts’ reaction is generally used to get. Bromide To Fluoride.
From testbook.com
Sodium Bromide Learn Definition, Properties, Structure & Uses Bromide To Fluoride There is one cobalt ion. Only one ion of each is needed. This is done by heating of the alkyl. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. Swarts reaction (also known as halogen exchange reaction or swarts fluorination reaction). Bromide To Fluoride.
From fumico.co.za
Sulfuryl Fluoride vs Methyl Bromide Performance and Efficacy Fumico Bromide To Fluoride There are two different types of reaction which might go on when concentrated sulphuric acid is added to a solid ionic halide like sodium fluoride, chloride, bromide or iodide. Only one ion of each is needed. This is done by heating of the alkyl. Swarts reaction (also known as halogen exchange reaction or swarts fluorination reaction) is a procedure that. Bromide To Fluoride.
From www.shutterstock.com
24 Silver Bromide Images, Stock Photos & Vectors Shutterstock Bromide To Fluoride There is one cobalt ion. This is accomplished by heating the alkyl chloride/bromide in the presence of a heavy metal fluoride (for example, silver fluoride or mercurous fluoride). There are two different types of reaction which might go on when concentrated sulphuric acid is added to a solid ionic halide like sodium fluoride, chloride, bromide or iodide. If we look. Bromide To Fluoride.
From www.fishersci.co.uk
Bromoacetyl bromide, 98, Thermo Scientific Chemicals Fisher Scientific Bromide To Fluoride Only one ion of each is needed. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. This is done by heating of the alkyl. Swarts’ reaction is generally used to get alkyl fluorides from alkyl chlorides or. Bromide To Fluoride.
From www.youtube.com
Sodium bromide YouTube Bromide To Fluoride If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. This is done by heating of the alkyl. There are two different types of reaction which might go on when concentrated sulphuric acid is added to a solid. Bromide To Fluoride.
From www.youtube.com
Ethene + Hydrogen Bromide (Reaction and Mechanism) YouTube Bromide To Fluoride There are two different types of reaction which might go on when concentrated sulphuric acid is added to a solid ionic halide like sodium fluoride, chloride, bromide or iodide. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1−. Bromide To Fluoride.
From www.chemkits.eu
Sodium bromide, 99.8+, 7647156 Bromide To Fluoride This is accomplished by heating the alkyl chloride/bromide in the presence of a heavy metal fluoride (for example, silver fluoride or mercurous fluoride). Only one ion of each is needed. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a. Bromide To Fluoride.
From www.bhphotovideo.com
Photographers' Formulary Potassium Bromide (100g) 100930 100G Bromide To Fluoride There are two different types of reaction which might go on when concentrated sulphuric acid is added to a solid ionic halide like sodium fluoride, chloride, bromide or iodide. There is one cobalt ion. This is done by heating of the alkyl. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the. Bromide To Fluoride.
From www.alamy.com
Glycopyrronium bromide (glycopyrrolate) COPD drug molecule. Has Bromide To Fluoride If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1− charge. Swarts’ reaction is generally used to get alkyl fluorides from alkyl chlorides or alkyl bromides. Only one ion of each is needed. There is one cobalt ion. This. Bromide To Fluoride.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Bromide To Fluoride This is done by heating of the alkyl. There is one cobalt ion. Only one ion of each is needed. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion. This is accomplished by heating the alkyl chloride/bromide in the presence of. Bromide To Fluoride.