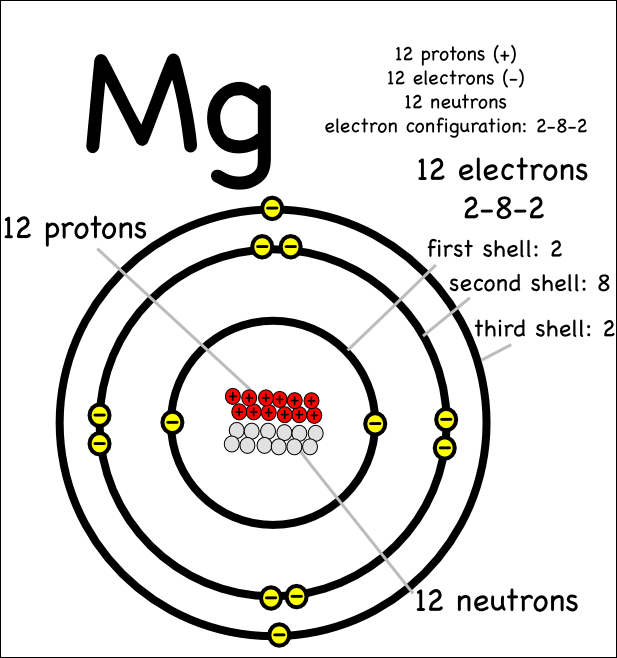
Magnesium Electron Shell Configuration . Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. The second electron shell plays a crucial role in determining the chemical. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Electron configuration chart of all elements is mentioned in the table below. Magnesium has two valence electrons in the 3s orbital. The shorthand electron configuration (or noble gas. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. Today we will tell you about the electron configuration of the mg. Here, electrons fill the 1s, 2s, and 2p. For magnesium (mg), which has an atomic number of 12, the electron configuration is: The electron configuration of magnesium is 1s2 2s2 2p6 3s2. Since 1s can only hold two electrons. The ninth most abundant element in the universe.
from montessorimuddle.org
The second electron shell plays a crucial role in determining the chemical. The shorthand electron configuration (or noble gas. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Electron configuration chart of all elements is mentioned in the table below. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. Here, electrons fill the 1s, 2s, and 2p. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. The electron configuration of magnesium is 1s2 2s2 2p6 3s2. For magnesium (mg), which has an atomic number of 12, the electron configuration is: Magnesium has two valence electrons in the 3s orbital.
subatomic particles Montessori Muddle
Magnesium Electron Shell Configuration Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas. The ninth most abundant element in the universe. The electron configuration of magnesium is 1s2 2s2 2p6 3s2. Since 1s can only hold two electrons. The second electron shell plays a crucial role in determining the chemical. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. Here, electrons fill the 1s, 2s, and 2p. Today we will tell you about the electron configuration of the mg. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Electron configuration chart of all elements is mentioned in the table below. For magnesium (mg), which has an atomic number of 12, the electron configuration is: Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Magnesium has two valence electrons in the 3s orbital.
From www.dreamstime.com
Model of magnesium atom stock vector. Illustration of mass 164475021 Magnesium Electron Shell Configuration Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. For magnesium (mg), which has an atomic number of 12, the electron configuration is: All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. The electron configuration of magnesium is. Magnesium Electron Shell Configuration.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Magnesium Electron Shell Configuration In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Here, electrons fill the 1s, 2s, and 2p. The electron configuration of magnesium is 1s2 2s2 2p6 3s2. The ninth most abundant element in the universe. Today we will tell you about the. Magnesium Electron Shell Configuration.
From nursehub.com
Electron Shells NurseHub Magnesium Electron Shell Configuration In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Electron configuration chart of all elements is mentioned in the table below. The electron configuration of magnesium is 1s2 2s2 2p6 3s2. Magnesium has two valence. Magnesium Electron Shell Configuration.
From montessorimuddle.org
subatomic particles Montessori Muddle Magnesium Electron Shell Configuration For magnesium (mg), which has an atomic number of 12, the electron configuration is: Here, electrons fill the 1s, 2s, and 2p. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Since 1s can only. Magnesium Electron Shell Configuration.
From utedzz.blogspot.com
Periodic Table Magnesium Atomic Number Periodic Table Timeline Magnesium Electron Shell Configuration Electron configuration chart of all elements is mentioned in the table below. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Today we will tell you about the electron configuration of the mg. Since 1s can. Magnesium Electron Shell Configuration.
From www.goodscience.com.au
Electron Configuration (Elements 120) Good Science Magnesium Electron Shell Configuration The electron configuration of magnesium is 1s2 2s2 2p6 3s2. The shorthand electron configuration (or noble gas. Today we will tell you about the electron configuration of the mg. The second electron shell plays a crucial role in determining the chemical. Magnesium has two valence electrons in the 3s orbital. The ninth most abundant element in the universe. Find the. Magnesium Electron Shell Configuration.
From www.numerade.com
SOLVED Base your answers to questions 3032 on the information below Magnesium Electron Shell Configuration Since 1s can only hold two electrons. The second electron shell plays a crucial role in determining the chemical. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. The. Magnesium Electron Shell Configuration.
From valenceelectrons.com
Electron Configuration for Magnesium(Mg, Mg2+ ion) Magnesium Electron Shell Configuration For magnesium (mg), which has an atomic number of 12, the electron configuration is: The shorthand electron configuration (or noble gas. Magnesium has two valence electrons in the 3s orbital. Electron configuration chart of all elements is mentioned in the table below. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. The. Magnesium Electron Shell Configuration.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Shell Configuration Electron configuration chart of all elements is mentioned in the table below. Today we will tell you about the electron configuration of the mg. The ninth most abundant element in the universe. For magnesium (mg), which has an atomic number of 12, the electron configuration is: Magnesium has two valence electrons in the 3s orbital. Find the full electronic configuration. Magnesium Electron Shell Configuration.
From www.newtondesk.com
Magnesium Mg (Elements 12) of Periodic Table Elements FlashCards Magnesium Electron Shell Configuration Here, electrons fill the 1s, 2s, and 2p. Since 1s can only hold two electrons. The second electron shell plays a crucial role in determining the chemical. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Electron configuration chart of all elements is mentioned in the table below. Find the full electronic. Magnesium Electron Shell Configuration.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image Magnesium Electron Shell Configuration The electron configuration of magnesium is 1s2 2s2 2p6 3s2. Since 1s can only hold two electrons. Today we will tell you about the electron configuration of the mg. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Here, electrons fill the. Magnesium Electron Shell Configuration.
From charlee-yersblogbarnes.blogspot.com
Atomic Mass of Magnesium Magnesium Electron Shell Configuration Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Since 1s can only hold two electrons. For magnesium (mg), which has an atomic number of 12, the electron configuration is: The second electron shell plays a crucial role in determining the chemical. The ninth most abundant element in the universe. Today we. Magnesium Electron Shell Configuration.
From www.thesciencehive.co.uk
Atomic Structure and Electron Configuration (AQA) — the science hive Magnesium Electron Shell Configuration Magnesium has two valence electrons in the 3s orbital. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. Today we will tell you about the electron configuration of the mg. The ninth most abundant element in the universe. Since 1s can only hold two electrons. Here,. Magnesium Electron Shell Configuration.
From commons.wikimedia.org
FileElectron shell 012 Magnesium.svg Wikimedia Commons Magnesium Electron Shell Configuration Since 1s can only hold two electrons. The shorthand electron configuration (or noble gas. The ninth most abundant element in the universe. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The electron configuration of magnesium. Magnesium Electron Shell Configuration.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Electron Shell Configuration Since 1s can only hold two electrons. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. The shorthand electron. Magnesium Electron Shell Configuration.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Shell Configuration Electron configuration chart of all elements is mentioned in the table below. The ninth most abundant element in the universe. For magnesium (mg), which has an atomic number of 12, the electron configuration is: Magnesium has two valence electrons in the 3s orbital. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator.. Magnesium Electron Shell Configuration.
From ar.inspiredpencil.com
Electron Configuration Of Magnesium Magnesium Electron Shell Configuration The second electron shell plays a crucial role in determining the chemical. Electron configuration chart of all elements is mentioned in the table below. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons. Today we will tell you about the electron configuration of the mg.. Magnesium Electron Shell Configuration.
From www.schoolmykids.com
Magnesium (Mg) Element Information, Facts, Properties, Uses Magnesium Electron Shell Configuration Magnesium has two valence electrons in the 3s orbital. Here, electrons fill the 1s, 2s, and 2p. The second electron shell plays a crucial role in determining the chemical. The electron configuration of magnesium is 1s2 2s2 2p6 3s2. Electron configuration chart of all elements is mentioned in the table below. Since 1s can only hold two electrons. All elements. Magnesium Electron Shell Configuration.
From valenceelectrons.com
Magnesium(Mg) electron configuration and orbital diagram Magnesium Electron Shell Configuration All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. Today we will tell you about the electron configuration of the mg. The shorthand electron configuration (or noble gas. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. For. Magnesium Electron Shell Configuration.
From www.youtube.com
Mg Orbital Diagram How to Write the Atomic Orbital Diagram for Magnesium Electron Shell Configuration The ninth most abundant element in the universe. The electron configuration of magnesium is 1s2 2s2 2p6 3s2. Electron configuration chart of all elements is mentioned in the table below. Today we will tell you about the electron configuration of the mg. Here, electrons fill the 1s, 2s, and 2p. Magnesium has two valence electrons in the 3s orbital. The. Magnesium Electron Shell Configuration.
From www.dreamstime.com
Electron of the Element Magnesium Stock Vector Illustration of Magnesium Electron Shell Configuration Since 1s can only hold two electrons. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. The ninth most abundant element in the universe. Today we will tell you. Magnesium Electron Shell Configuration.
From www.mikrora.com
File Electron Configuration Magnesium Svg Best Diagram Collection Magnesium Electron Shell Configuration All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. The second electron shell plays a crucial role in determining the chemical. The ninth most abundant element in the universe. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator.. Magnesium Electron Shell Configuration.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Electron Shell Configuration In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Here, electrons fill the 1s, 2s, and 2p. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. Today we will tell you about the electron configuration of the mg.. Magnesium Electron Shell Configuration.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Magnesium Electron Shell Configuration Magnesium has two valence electrons in the 3s orbital. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. The ninth most abundant element in the universe. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Since 1s can. Magnesium Electron Shell Configuration.
From sciencenotes.org
Magnesium Atom Science Notes and Projects Magnesium Electron Shell Configuration Since 1s can only hold two electrons. Today we will tell you about the electron configuration of the mg. The second electron shell plays a crucial role in determining the chemical. The electron configuration of magnesium is 1s2 2s2 2p6 3s2. The shorthand electron configuration (or noble gas. For magnesium (mg), which has an atomic number of 12, the electron. Magnesium Electron Shell Configuration.
From ar.inspiredpencil.com
Magnesium Atom Structure Magnesium Electron Shell Configuration Here, electrons fill the 1s, 2s, and 2p. The ninth most abundant element in the universe. For magnesium (mg), which has an atomic number of 12, the electron configuration is: Electron configuration chart of all elements is mentioned in the table below. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. The. Magnesium Electron Shell Configuration.
From ar.inspiredpencil.com
Magnesium Atom Diagram Magnesium Electron Shell Configuration The second electron shell plays a crucial role in determining the chemical. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Electron configuration chart of all elements is mentioned in the table below. Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. The electron configuration of magnesium is 1s2 2s2 2p6. Magnesium Electron Shell Configuration.
From www.pinterest.com
Magnesium, atomic structure Stock Image C018/3693 Science Photo Magnesium Electron Shell Configuration In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. The ninth most abundant element in the universe. Today we will tell you about the electron configuration of the mg. Here, electrons fill the 1s, 2s, and 2p. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Electron configuration chart of all. Magnesium Electron Shell Configuration.
From www.shutterstock.com
Magnesium Atom. Diagram Representation Of The Element Magnesium Magnesium Electron Shell Configuration The second electron shell plays a crucial role in determining the chemical. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Magnesium has two valence electrons in the 3s. Magnesium Electron Shell Configuration.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Electron Shell Configuration The second electron shell plays a crucial role in determining the chemical. Here, electrons fill the 1s, 2s, and 2p. Today we will tell you about the electron configuration of the mg. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. In writing the electron configuration. Magnesium Electron Shell Configuration.
From www.alamy.com
3d render of atom structure of magnesium isolated over white background Magnesium Electron Shell Configuration Here, electrons fill the 1s, 2s, and 2p. The shorthand electron configuration (or noble gas. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Magnesium has two valence electrons in the 3s orbital. In writing the electron configuration for magnesium the first. Magnesium Electron Shell Configuration.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Shell Configuration Since 1s can only hold two electrons. The ninth most abundant element in the universe. Today we will tell you about the electron configuration of the mg. Here, electrons fill the 1s, 2s, and 2p. Electron configuration chart of all elements is mentioned in the table below. The electron configuration of magnesium is 1s2 2s2 2p6 3s2. In writing the. Magnesium Electron Shell Configuration.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Shell Configuration The ninth most abundant element in the universe. For magnesium (mg), which has an atomic number of 12, the electron configuration is: All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. The electron configuration of magnesium is 1s2 2s2 2p6 3s2. Here, electrons fill the 1s,. Magnesium Electron Shell Configuration.
From www.nagwa.com
Question Video Identifying the Energy Level Diagram That Represents Magnesium Electron Shell Configuration Magnesium has two valence electrons in the 3s orbital. The electron configuration of magnesium is 1s2 2s2 2p6 3s2. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. Today we will tell you about the electron configuration of the mg. Here, electrons. Magnesium Electron Shell Configuration.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Magnesium Electron Shell Configuration Electron configuration chart of all elements is mentioned in the table below. All elements of group 2 have the same configuration of an electron in the outer electron shell and also a similar crystal structure. For magnesium (mg), which has an atomic number of 12, the electron configuration is: Today we will tell you about the electron configuration of the. Magnesium Electron Shell Configuration.