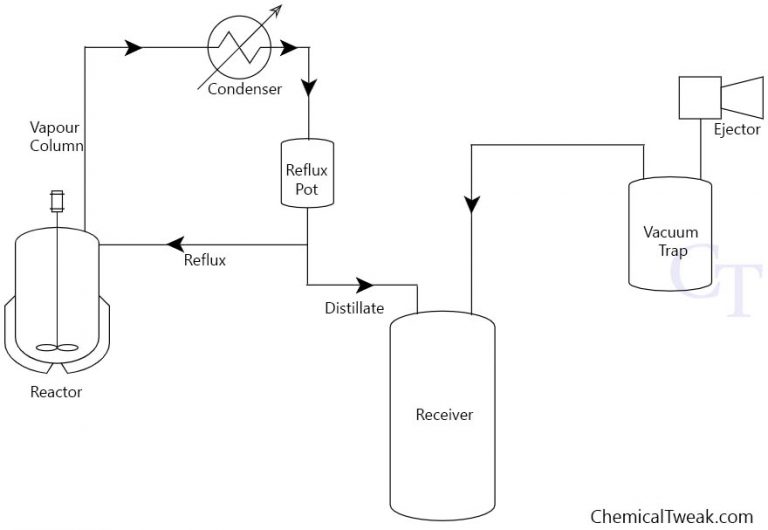
Function Of Vacuum Distillation . A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. This is particularly useful for distilling products of boiling points over 100oc. Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling point but which are. When the pressure is lowered inside. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup.
from chemicaltweak.com
Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling point but which are. When the pressure is lowered inside. Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. This is particularly useful for distilling products of boiling points over 100oc. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure.
Vacuum Distillation Process And Working Principle VDU Full Form
Function Of Vacuum Distillation Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling point but which are. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. This is particularly useful for distilling products of boiling points over 100oc. When the pressure is lowered inside. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure.
From www.researchgate.net
Schematic Process Flow Diagram for Vacuum Distillation Download Function Of Vacuum Distillation This is particularly useful for distilling products of boiling points over 100oc. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a distillation carried out at reduced pressure to. Function Of Vacuum Distillation.
From chemicaltweak.com
Vacuum Distillation Process And Working Principle VDU Full Form Function Of Vacuum Distillation A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with. Function Of Vacuum Distillation.
From www.vecteezy.com
Distillation process diagram for education 3227893 Vector Art at Vecteezy Function Of Vacuum Distillation Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. When the pressure is lowered inside. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48).. Function Of Vacuum Distillation.
From www.theengineeringconcepts.com
Types of Distillation The Engineering Concepts Function Of Vacuum Distillation When the pressure is lowered inside. This is particularly useful for distilling products of boiling points over 100oc. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is a technique for the purification or separation of. Function Of Vacuum Distillation.
From www.researchgate.net
The schematic for the vacuum distillation process. Download Function Of Vacuum Distillation This is particularly useful for distilling products of boiling points over 100oc. When the pressure is lowered inside. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a distillation carried out at reduced pressure to. Function Of Vacuum Distillation.
From www.researchgate.net
Flow diagram of a dry vacuum distillation unit [9] Download Function Of Vacuum Distillation This is particularly useful for distilling products of boiling points over 100oc. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling point but which are. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple. Function Of Vacuum Distillation.
From www.researchgate.net
Process flow diagram of Vacuum Distillation Unit Download Scientific Function Of Vacuum Distillation A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. This is particularly useful for distilling products of boiling points over 100oc. Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is. Function Of Vacuum Distillation.
From www.pinterest.com
Simple Distillation Distillation, Chemistry lessons, Teaching chemistry Function Of Vacuum Distillation Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling point but which. Function Of Vacuum Distillation.
From schlenklinesurvivalguide.com
Static Vacuum Distillation The Schlenk Line Survival Guide Function Of Vacuum Distillation A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. When the pressure is lowered inside. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. This is particularly useful for distilling products of boiling points over 100oc. A vacuum distillation is performed by applying a vacuum source to. Function Of Vacuum Distillation.
From chem.libretexts.org
5.4C StepbyStep Procedures for Vacuum Distillation Chemistry Function Of Vacuum Distillation This is particularly useful for distilling products of boiling points over 100oc. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a. Function Of Vacuum Distillation.
From ar.inspiredpencil.com
Batch Flow Vacuum Distillation Setup Function Of Vacuum Distillation Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling point but which are. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. Boiling commences. Function Of Vacuum Distillation.
From wintek-corp.com
Vacuum Distillation Vacuum Distillation Equipment Wintek Function Of Vacuum Distillation Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling point but which are. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is a distillation carried out at reduced pressure to lower boiling. Function Of Vacuum Distillation.
From www.alamy.com
Vacuum Distillation System Stock Vector Image & Art Alamy Function Of Vacuum Distillation A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with. Function Of Vacuum Distillation.
From www.wermac.org
Distillation Column Basic Distillation Equipment and Operation Function Of Vacuum Distillation When the pressure is lowered inside. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48).. Function Of Vacuum Distillation.
From en.wikipedia.org
Distillation Wikipedia Function Of Vacuum Distillation This is particularly useful for distilling products of boiling points over 100oc. When the pressure is lowered inside. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower. Function Of Vacuum Distillation.
From www.researchgate.net
Schematic of the vacuum distillation process (8 × 10⁴ Pa). Download Function Of Vacuum Distillation When the pressure is lowered inside. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. This is particularly. Function Of Vacuum Distillation.
From exonvyzbm.blob.core.windows.net
Vacuum Distillation Manufacturing Process at Brian Rose blog Function Of Vacuum Distillation Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling point but which are. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied. Function Of Vacuum Distillation.
From www.scribd.com
Distillation Equipment Parts and Functions Distillation Physical Function Of Vacuum Distillation A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150. Function Of Vacuum Distillation.
From micoope.com.gt
Distillation Apparatus Diagram With Full Process And Lab, 44 OFF Function Of Vacuum Distillation Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling point but which are. When the pressure is lowered inside. Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. Boiling commences when the vapor pressure of a liquid or. Function Of Vacuum Distillation.
From www.shemmassianconsulting.com
Reactions and Separations for the MCAT Everything You Need to Know Function Of Vacuum Distillation Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling point but which are. When the pressure is lowered inside. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). Vacuum. Function Of Vacuum Distillation.
From mavink.com
Vacuum Distillation Set Up Function Of Vacuum Distillation This is particularly useful for distilling products of boiling points over 100oc. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a technique for the purification or separation of. Function Of Vacuum Distillation.
From www.gustawater.com
The Ultimate Guide to Distillation and Distillation Columns Function Of Vacuum Distillation A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. This is particularly useful for distilling products of boiling points over 100oc. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling point but which are. Boiling commences when the. Function Of Vacuum Distillation.
From www.researchgate.net
Vacuum distillation apparatus. Download Scientific Diagram Function Of Vacuum Distillation When the pressure is lowered inside. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. This is particularly useful for distilling products of boiling points over 100oc. A vacuum distillation apparatus is. Function Of Vacuum Distillation.
From www.britannica.com
distillation summary Britannica Function Of Vacuum Distillation A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). This is particularly useful for distilling products of boiling points over 100oc. Vacuum distillation is a technique for the purification or separation of. Function Of Vacuum Distillation.
From www.researchgate.net
Schematic Process Flow Diagram for Vacuum Distillation Download Function Of Vacuum Distillation When the pressure is lowered inside. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling point but which are. Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. A vacuum distillation is performed by applying a vacuum source. Function Of Vacuum Distillation.
From chemicaltweak.com
Vacuum Distillation Process And Working Principle VDU Full Form Function Of Vacuum Distillation This is particularly useful for distilling products of boiling points over 100oc. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. When the pressure is lowered inside. Vacuum distillation is a distillation carried out at reduced pressure to. Function Of Vacuum Distillation.
From www.processingmagazine.com
Controlling chemical vacuum processes with direct sealing diaphragm Function Of Vacuum Distillation A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). This is particularly useful for distilling products of boiling points over 100oc. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower. Function Of Vacuum Distillation.
From www.simplepharmanotes.com
Distillation under reduced pressure / Vacuum Distillation. Function Of Vacuum Distillation A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. When the pressure is lowered inside. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Boiling commences when. Function Of Vacuum Distillation.
From www.youtube.com
Distillation Process Vacuum Distillation Process Distillation Function Of Vacuum Distillation Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. When the pressure is lowered inside. This is particularly useful for distilling products of boiling points over 100oc. A vacuum distillation apparatus is shown in figure 5.50, using a. Function Of Vacuum Distillation.
From www.youtube.com
Vacuum Distillation YouTube Function Of Vacuum Distillation A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. This is particularly useful for distilling products of boiling points over 100oc. Vacuum distillation is a technique for the purification or separation of. Function Of Vacuum Distillation.
From foodtechnotes.com
Distillation Principle and Types Food Tech Notes Function Of Vacuum Distillation Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling point but which are. When the pressure is lowered inside. This is particularly useful for distilling products of boiling points over 100oc. Vacuum distillation is a distillation carried out at reduced pressure to lower. Function Of Vacuum Distillation.
From www.slideserve.com
PPT Chapter 4 Crude distillation PowerPoint Presentation, free Function Of Vacuum Distillation When the pressure is lowered inside. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. This is particularly useful for distilling products of boiling points over 100oc. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter. Function Of Vacuum Distillation.
From www.researchgate.net
The vacuum distillation apparatus scheme. Download Scientific Diagram Function Of Vacuum Distillation A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. When the pressure is lowered inside. This is particularly useful for distilling products of boiling points over 100oc. Vacuum distillation is a distillation carried out at reduced pressure to. Function Of Vacuum Distillation.
From www.theengineersperspectives.com
Vacuum Distillation System The Engineer's Perspective Function Of Vacuum Distillation Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling point but which are. When the pressure is lowered inside. A vacuum distillation apparatus is shown in figure 5.50, using. Function Of Vacuum Distillation.
From www.pressurecontrolsolutions.com
Vacuum Distillation issues? Call Pressure Control Solutions! Function Of Vacuum Distillation A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. When the pressure is lowered inside. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is a distillation carried out at reduced pressure to lower boiling points. Vacuum distillation is a technique for the purification or. Function Of Vacuum Distillation.